This web page was produced as an assignment for an undergraduate course at Davidson College
Article Summary: A Systems Biology Approach Exposes Risks of Lansoprazole in the Pathogenesis of Alzheimer’s Disease
Article Summary:
In the article “The proton-pump inhibitor lansoprazole enhances amyloid beta production” Badiola et al. (2013) present in vitro and in vivo evidence that a commonly prescribed proton-pump inhibitor (PPI) alters the profile of amyloid-β species in Alzheimer’s disease. Accumulation of various amyloid-β (Aβ) peptides is implicated in Alzheimer’s disease (AD) pathogenesis. Badiola et al. decided to investigate lansoprazole after in silico investigations using a therapeutic performance mapping system identified the drug as a possible modulator of amyloid plaque formation.
In vitro investigations of lansoprazole and three other PPIs showed that cellular AD models produced elevated levels of Aβ40 and Aβ42 in response to drug treatment. An in depth mass spectrometry analysis of Aβ species after lansoprazole treatment revealed that this drug may act as an inverse γ-secretase modulator (iGSM). Inverse GSMs reduce relative Aβ38 and increase relative Aβ42 production by altering the sites at which γ-secretase cleaves pre-Aβ species to form Aβ peptides. In these same cells, further analyses of Aβ formation enzymes suggested elevated activity, but unchanged protein expression, of another the enzyme BACE1. Combined, these findings offered explanations for elevated levels of many Aβ peptides (but reduced Aβ38) in response to lansoprazole treatment in vitro.
The in vivo portion of the Badiola et al. study exposed mouse models to lansoprazole treatment and subsequently quantified Aβ peptide species in brain tissue. These analyses revealed significant increases in Aβ40 and non-significant increases in Aβ42 for the treated mice. Importantly, these results confirmed that lansoprazole can modulate Aβ formation in vivo. Badiola et al. conclude their study by proposing a model for lansoprazole-induced changes in Aβ production incorporating the drug’s amplification of BACE1 activity, and its iGSM role. Overall, results from this study propose potential contra-indications for PPIs in Alzheimer’s disease.
Article Analysis:
This study was well designed in that the authors took advantage of a computer model generate a testable hypothesis about AD and subsequently used experimental data to refine their models of AD drug interactions. Badiola et al. began with model-based questions of whether and which Aβ species would be affected by lansoprazole treatments. Once these questions were answered, the investigators dug deeper for the mechanistic explanations which allowed them to refine the model.
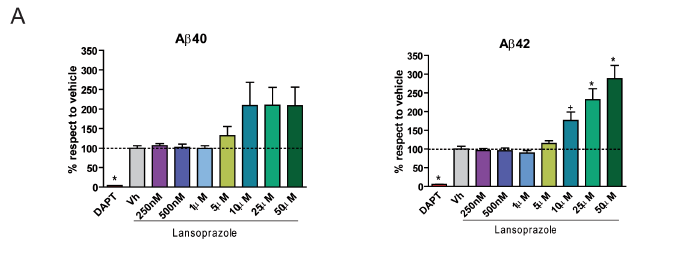
However, one of the parts of the study that caused concern was their description of Aβ40 trends in Figure 1A. The authors cite the doubling of Aβ40 at certain levels of lansoprazole exposure, but in fact their results here are not statistically significant. Likewise, increases in Aβ40 are not clearly apparent in the mass spectrophotometry analysis (Figure 2A) although the authors cite a slight increase here also. Although these assertions about Aβ40 levels are consistent with the authors’ ultimate model and the in vivo analyses, they also seem to misrepresent the in vitro data.
Figure Summaries:


Figure 1.
A) Authors investigated in vitro effects of lansoprazole treatments using a transgenic Chinese hamster ovary cell model of AD. For 24 hours cells were exposed to variable concentrations of lansoprazole solution, the control (vehicle, Vh) solution, or a negative DATP control solution(n=6 for each trial). DATP acted as a negative control because it inhibits activity of the Aβ-forming enzyme γ-secretase. In each case, Aβ levels in the cell media were quantified using either a human amyloid-β kit (Aβ42) of ELISA (Aβ40). Aβ levels were graphed as a percent of the mean vehicle control level (also represented by the dotted lline) and error bars represent standard deviation. For Aβ40 investigations, only the negative control differed significantly from the vehicle control (*, p<0.01). For Aβ42 investigations, DATP was significantly lower than the control and lansoprazole treatments from 10-50 μM were significantly higher (+, p<0.05; *, p<0.01).
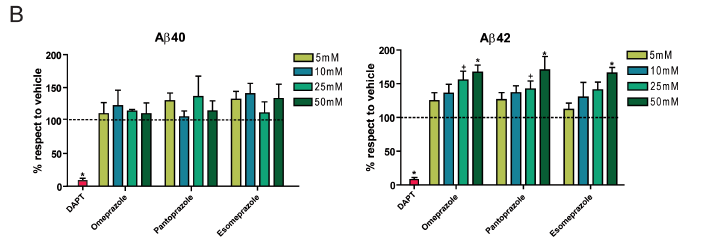
B) Authors investigated in vitro effects of three other PPIs (omeprazole, pantoprazole, and esomeprazole) using procedures as described in A), but for a smaller range of drug concentrations. For Aβ40, authors describe Aβ increases as “non-homogenous,” and indeed no significant Aβ40 increases were observed in these drug treatments. By contrast, all three treatments exhibited significantly higher Aβ42 production at high drug concentrations (+, p<0.05; *, p<0.01). DATP treatment caused significantly reduced Aβ production for both species (*, p<0.01). By comparing these results to A) it is possible to see similarities among the drug effects of various PPIs in the AD model system.
Figure reprinted under a Creative Commons license from Badiola et al. 2012.
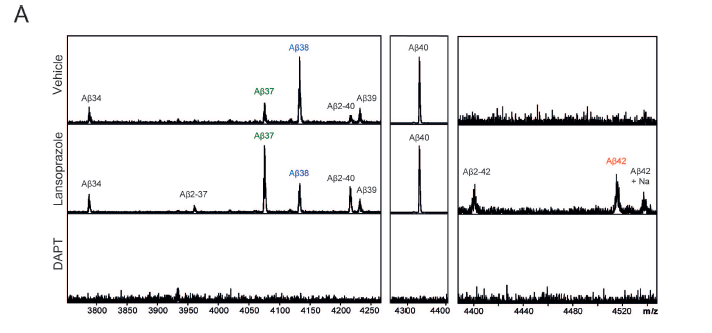
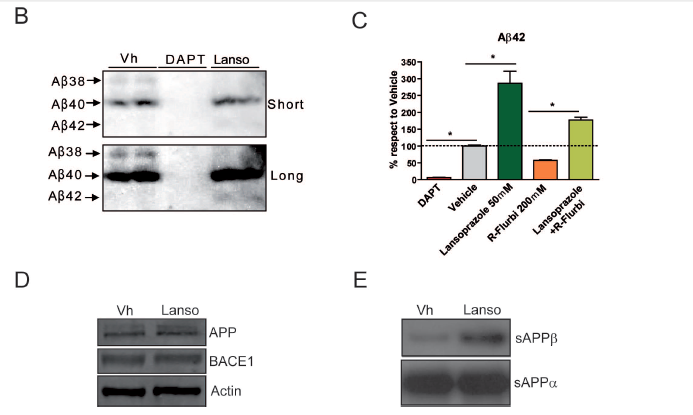
Figure 2.
A) A mass spectrometry analysis following immunoprecipitation of culture media from control (vehicle), Lansaprazole, and DATP treated model cells reveals differences in Aβ peptide composition. The lansoprazole treatment cells were exposed to 50 μM of the drug for 24 hours. This treatment exhibits elevated Aβ37 and Aβ42 and decreased Aβ38 as compared with the vehicle control, as well as elevated Aβ2-37, Aβ2-40, and Aβ2-42. Although the authors' results also cite elevated Aβ40, this increase is not apparent in the graph. As expected, no Aβ species were observed in the DATP treatment. Other peptide composition changes are explored in later figure components, but authors posit that Aβ2 changes may relate to differential meprin β metalloprotease activity when media pH changes (as in PPI treatment).
Badiola et al. explore elevated Aβ42 and decreased Aβ38 as related to iGSM activity of the lansoprazole in figure components B and C. B) displays western blot analysis for three different Aβ peptides (Aβ42, Aβ40, and Aβ38) for the treatment groups described in A). The images confirm the conclusion from A) that Aβ42 increases and Aβ38 decreases with Lansoprazole treatment. The top and bottom panels show the same images at different (short vs. long) exposures so that lighter (Aβ38) blots appear more clearly in the bottom panel.
C) This figure supports the hypothesis that lansoprazole acts as an iGSM because it restores higher Aβ42 formation in cells treated with the straight GSM drug R-flurbiprofen. Straight GSMs and iGSMs have opposite effects on relative Aβ peptide production with GSMs elevating Aβ38 and decreasing Aβ42. The figure again shows positive (vehicle) and negative (DAPT) controls, and the compares Aβ42 levels, as determined by ELISA immunoassays, in three drug treatment groups (lansaprazole at 50 μM, R-flurbiprofen at 200 μM, and combination treatment), as a percentage of lvels detected in the vehicle control (dotted line). Lansoprazole treated Aβ42 levels were significantly elevated as compared to the control, likewise combined treatment Aβ42 levels were significantly elevated as compared to levels in R-flurbiprofen treatment (*, p<0.01). This restoration of Aβ42 levels strongly supports the authors' iGSM hypothesis.
D) Demonstrates via a western blot analysis that lansaprazole treatment does not affect protein expression levels for the pre-Aβ peptide APP or for the Aβ-generating enzyme BACE1. This suggests that elevated Aβ peptides in lansoprazole treatment cannot be attributed to elevated levels of pre-Aβ species or to the quantity of this converting enzyme.
E) Authors diplay western blot analyis showing elevated levels of the pre-Aβ peptide sAPPβ with lansoprazole treatment, but no change in protein levels of another pre-Aβ peptide sAPPα. Two possible enzymes may act on APP, one (BACE1) forms sAPPβ and the other forms sAPPα. Only sAPPβ goes on to form Aβ peptides. Thus, the western blot suggests that increased BACE1 activity may contribute to the elevated Aβ peptides levels in lansoprazole treatments.
Figure reprinted under a Creative Commons license from Badiola et al. 2012.
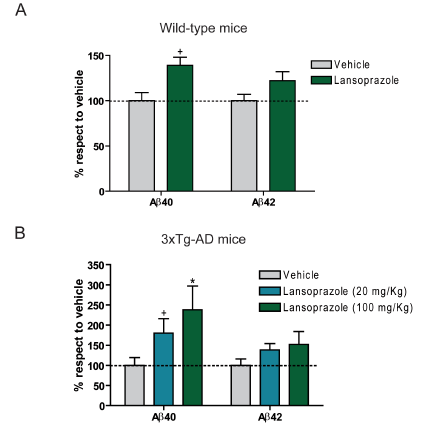
Figure 3.
A) Non-transgenic mice (n= 10) were exposed to a lansoprazole of 100 mg/kg for 5 days and homogenized brain tissue was later analyzed by ELISA for two amyloid peptides: Aβ40 and Aβ42. Statistically higher (+, p<0.05) levels of Aβ40 were observed in the lansoprazole treatment group as compared to the untreated control (vehicle) group.
B) Transgenic mice (3xTg-AD; n= 6) whose genetic alterations cause age-dependent amyloid plaque formation were treated with lansoprazole at two different concentrations for 5 days. Brain tissue was analyzed as in A). Statistically higher (+, p<0.05; *, p<0.01) levels of Aβ40 were observed in the both lansoprazole treatment groups as compared to the vehicle group. Notably, the 20 mg/Kg treatment is comparable to human prescribed doses of lansoprazole
A) and B) together indicate that lansoprazole is indeed able to affect Aβ production in vivo. None of these data indicate significant changes in Aβ42 levels, contrary to in vitro findings. Researchers attribute this difference partly the way Aβ was quantified; in vivo measurements included intracellular peptides while in vitro measurements only observed extracellular Aβ.
Figure reprinted under a Creative Commons license from Badiola et al. 2012.
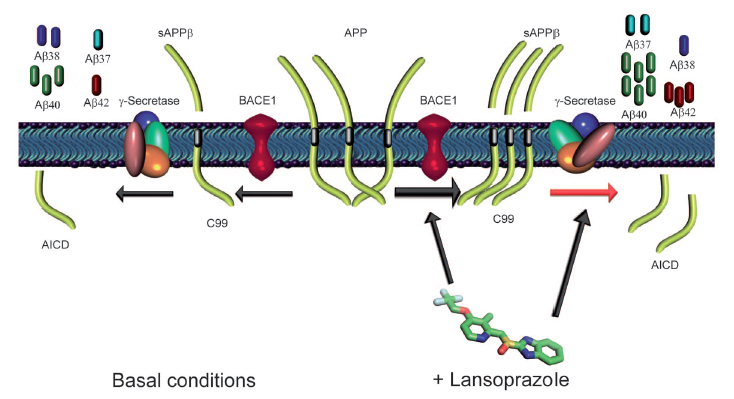
Figure 4.
This figure summarizes the authors’ proposed model for lansoprazole modulation of Aβ production in AD. The left side of the panel shows normal Aβ generation in the absence of the drug. Initially, APP molecules are cleaved by BACE1 protease to form soluble APPβ and a C99 carboxyl terminal fragment. Subsequently, γ-secretase cleaves the C99 fragment, releasing Aβ peptides from the cell and retaining the amyloid precursor intracellular domain (AICD) within the cell. In the lansoprazole treated right side of the panel BACE1 activity is elevated (generating more sAPPβ/c99) and γ-secretase activity is altered by lansoprazole’s iGSM properties (increasing Aβ42, but decreasing Aβ38). Thus lansoprazole is able to increase production of Aβ37, Aβ40, and Aβ42 but decrease the production of Aβ38.
Figure reprinted under a Creative Commons license from Badiola et al. 2012.
Works Cited