This page was created as an assignment for an undergraduate class at Davidson College.
Chronic Inflammatory Demyelinating Polynueropathy (CIDP)
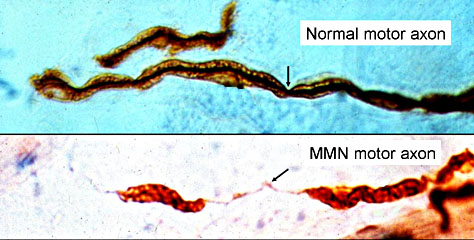
Figure 1: This illustrating shows nerve demyelination in a disorder similar to CIDP: Multifocal Motor Neuropathy (MMN).
Myelinated Axons: Segmental demyelination in Multifocal Motor Neuropathy (MMN)
Permission to use this figure was requested from Washington University. If permission is denied the figure will be removed.(http://www.neuro.wustl.edu/neuromuscular/antibody/pnimdem.html)
Introduction to Autoimmunity:
Autoimmune disease occurs when the immune system initiates a specific adaptive response to a self protein (Janeway, 2001). They are a direct result of the open range of specificity existing on B-cell and T-cell receptors allowing them to recognize any antigen entering the body. This wide range of recognization comes at a small price, due to the mechanisms in place to prevent self-reactivity. The result of this specificity is contra-indicated only if something goes wrong in the normal pathway. This can occur through several methods: The method hypothesized to be at work in CIDP is the representation of self peptide as well as antigen peptide on a cell's surface prompting the survival signal for lymphocytes and allows them to make antibodies and T-cells specific for the infection as well as the self-protein.
Once autoimmunity has been established, the ongoing problem of the inability for the immune system to shut off the self-reactive response because the effector mechanisms cannot be completely eliminated creates a chronic problem. In CIDP, however, there is no indication that persistent antigen stimulation is related to the chronic nature of the disease ( van Doorn, 2000). The result of the constant activation of effector pathways is chronic inflammation which can prove lethal to affected tissues (Janeway 2001).
Introduction to CIDP:
CIDP is an autoimmune disease, which affects the motor and sensory nerves by destroying the myelin sheaths surrounding the nerves. The myelin sheath is a fatty covering acting as an insulator to the fibers of nerves (NINDS, 2001). Without this covering, nerves incur greater damage with regular activity. There is strong evidence that the inflammation in the neural tissue is what causes the damage to the sheath and ultimately the nerves (Guillain-Barre Syndrome Support Group). This inflammation is macrophage mediated (Washington University). CIDP is thought to be the chronic variety of Guillain-Barre Syndrome (GBS), which is an acute immune mediated polyneuropathy (van Doorn). There are two types of CIDP: chronic progressive, and relapsing. In chronic progressive patients, the disease gradually worsens over time. Relapsing patients show and ebb and flow of symptoms slipping in and out of remission over the course of their disease. The classification of CIDP does not typically change the course of treatment, but has some effect on the success of the treatment modalities (Washington University, 2003).
This disease is characterized by progressive weakness and loss of neurological function primarily in the legs and arms (NINDS, 2001). Most patients with this disorder present with a case of progressive weakness for at least two months (van Doorn, 2000), the temporal indicator of the chronic form of this neuropathy. The course of the disease tends to be either slow and gradual, or characterized by periods of relapses and remissions (van Doorn, 2000). Although this disease is slightly more prevalent in young adults, it can occur at any age, and statistics show that men are twice as likely as women to contract this disease (NINDS, 2001). Some typical symptoms include: symmetric tingling in the proximal and distal extremities, paralysis of the muscles, progressive weakness, sensory loss, loss of nero-muscular reflexes, and frequent loss of balance. It is not uncommon for a CIDP patient to to lose the ability to feel the sharp sensation of a pin stick in the affected area.This onset is prolonged and gradual over the course of at least 8 weeks (Koski, 2002). Statistics show that 70% of patients diagnosed with CIDP presenting only sensory symptoms develop motor symptoms over the course of 2-3 years (Koski, 2002).
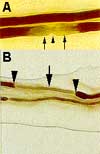
Note: Permission to use this figure has been requested from the Department of Neurology at Baylor College of Medicine. If permission is denied the figure will be removed.
Figure 2: This figure shows a representation of a demyelinated nerve
A. Demyelination at the paranodal region (solid arrow). Arrow head points to the node of Ranvier.
B. Demyelination of a nerve segment (solid arrow) between the two nodes of Ranvier (arrow heads). This finding is frequently observed in the nerve biopsy of CIDP.
The main reason CIDP is considered an immune disorder
is that it responds to immune-mediated treatment (Koski, 2002). It is very
difficult to diagnose
and can be diagnosed through a number of methods which are discussed
below. Although the aetiology remains unknown, both humoral and
cell-mediated immune mechanisms contribute to the pathogenesis ( Mahad, et
al, 2002).Immune or inflammatory mechanisms are thought to be the root cause
of many disorders
that, like
CIDP, reflect
damage
to
the
peripheral
nerves
and
affect
sensory function.
This turns out to be the most definite role of the immune system
in the development of CIDP; the mutation of the macrophage to cause
macrophage-mediated inflammation,
ultimately resulting in the demyelination of the nerves.
Another characteristic of CIDP, which leads to its classification
as an autoimmune disorder, is the evidence of preexisting infections
immediately
prior to the
development of the disease in a significant number of patients.
Studies show that in 70% of GBS patients (Guillain-Barre Syndrome), the acute
form of CIDP, have pre-existing infections prior to the development of symptoms.
Although the pathology of the infections preceding CIDP are studied less
frequently, statistics show that at the very least relapses are strongly
correlated with infection (van Doorn, 2000).
The specific pathway for induction of the autoimmunity associated with CIDP is unknown. The current research cannot eliminate the possibility of an antibody mediated self-reactivity or a T-cell mediated self reactivity (van Doorn, 2000). The possibility that infection is a possible stimulus to the development of CIDP can support either pathway (Janeway, 2001). Infections are capable of inducing co-stimulatory activity on antigen presenting cells that present low levels of the specific peptide being attacked, in this case myelin. Another pathway is that of molecular mimicry. In this situation, antibodies or T-cells generated in response to an infectious agent cross-react with self antigens (Janeway, 2001). A molecular mimic is a foreign antigen closely related in structure to a self peptide allowing an antibody mediated response mounted toward the mimicked peptide to also effect the self-protein (Janeway, 2001).
What components of the immune system are involved?
T-Cells:
The most widely explained mechanism for the demyelination of nerve cells in CIDP is a macrophage-mediated inflammatory response.Endoneurial inflammatory changes with T cell infiltrates and macrophage associated demyelination are present on nerve biopsies in patients in the acute phase of CIDP (Mahad, et al, 2002). These endoneurial lymphocytes are associated with the increase expression of mRNA for Tumor Necrosis Factor alpha (TNF alpha), Interferon gamma (INF gamma) and Interluekin-2 (IL-2) (Mahad, et al, 2002).. TNF alpha is involved in local inflammation and endothelial activation. INF gamma causes macrophage activation, increased expression of MHC molecules and Ig class switching. INF gamma also suppresses the less damaging TH2 response in favor of the inflammation mediation of TH1. IL-2 is a crucial chemokines for the adaptive immune response and aids in the proliferation of T-cells. All of these proteins are either produced by T-cells, Natural Killer Cells or Macrophages, or add to the existence of T-cells and cause the initial inflammatory response associated with infection (Janeway, 2001). Activated T lymphocytes are increased in the peripheral blood of CIDP patients. This diagnostic evidence coupled with the relative success of T-cell suppressive treatment makes the case for a possible malfunction in the TH1 inflammatory response.
Antibodies and Complement:
These components of the immune system play a role in the pathogenesis of CIDP in the presence of a blood-nerve barrier breakdown. (after the demyelination has begun)
The current research debates whether CIDP is associated with a specific antibody response against neural antigens (van Doorn, 2000). Antibodies against selected neuroblastoma cells have been found in 40% of patients with CIDP and GBS in comparison with only 4% in other neuropathies (van Doorn, 2000). This, however, is far from conclusive as to the direct pathology of the disease, as other groups show no antibody interaction with myelin proteins or glycolipid cell-surface receptors (van Doorn, 2000).
Some forms of CIDP can be diagnosed using autoantibody serums to tubulin. Tubulin is a molecule which mediates many cell processes specifically cell division and movement of materials within cells. The suggested mechanism for the autoantibody to tubulin involves both IgG and IgM antibodies capable of binding to the tubulin epitope. This pathology was present in 10% of patients with CIDP (Washington University, 2003).
Another specific immunologic interference occurring in 26% of patients with CIDP is an interaction between IgG antibodies and Schwann cell processes. Schwann cells are the cells that wrap themselves around nerves making a protective barrier for the nerve fibers. Schwann cells are the individual cells making up the myelin protective sheaths. This suggests that a patient presenting with symptoms of CIDP could possibly have an autoantibody response against cell schwann cells. This response tags the self-protein for natural killer cell destruction.
What are the current treatments?
Only a few controlled studies have been performed in CIDP. There is limited consistent evidence of the relative success of any of the major treatments. Since each person is different and likely has a slightly different form of the disease, treatments are tailored to the needs of the patient (van Doorn, 2000). Aside from steroid treatment, therapies for CIDP are extremely costly.
Neutralization of chemokines with anti-chemokine antibodies has been effective in reducing the level of inflammation.
Possible Targets:
CXCL9 (Monokine induced by ING gamma)
CXCL10 (INF gamma inducible protein)
CCL3 (macrophage inflammatory protein 1 alpha)
Corticosteroids:
What are they?
Powerful anti inflammatory agents used to suppress the harmful effects of immune responses. These drugs are derivatives of members of the glucocorticoid family of steroid hormones (Janeway, 2001). Upon binding to hormones, these drugs effectively regulate the transcription of specific genes (Janeway, 2001).
Most commonly used:
Prednisone- synthetic analog of cortisol
Problems:
Steroids can regulate up to 1% of genes in the genome (Janeway, 2001). This causes a problem when genes that are not part of the disease pathology are effected by the drug.
Intravenous Immunoglobulin (IvIg): The mechanism for this treatment is not clear, nor is the reason some patients respond and others do not.
What is it?
IvIg is the administration of foreign immunoglobulin to patients with autoimmune disorders. Often this therapy is preferred because of the safety and efficacy compared to alternative treatments. IvIg is prepared from human plasma and is usually pooled from approximately 3,000 to 10,000 donors (Brannagan, 2002). Typically the formulation contains more than 95% IgG and less than 2.5% IgA. Other plasma components such as CD4 (co-receptor for MHC II allowing induction of TH2 response), CD8 (co-receptor for MHC I allowing the induction of TH1 response), human leukocyte antigen (HLA) molecules, cytokines and coagulation factors may be present and may result in some of the negative side-effects (Brannagan, 2002). Maintenance of the therapy is needed to continue progression or prevent relapses (Brannagan, 2002).
Proposed action: (Ochi, et al, 2003).
Fc Receptor blockade: located on the C terminal of the Antibody these receptors aid in the initiation of effector cell responses by linking antibody binding to the effector cell function.
Competitive inhibition of C3b complement pathways: This molecule is the principle effector molecule of the complement system. Creates a blockage of complement response.
Down Regulation of B-and T-cell functions: causes an overall decrease in immune system function
Anti-idiotype suppression: suppression of the binding site of antibodies
Anti-cytokine effects: inhibits some of the homing devices for effector cells and reduces inflammation
Plasma Exchange:
What is it?
This is an apheresis procedure consisting of the separation of blood parts into red blood cells and plasma cells(http://hemato.unice.fr/sanderson/pe.htm). Typically the blood volume is balanced by replacement fluids containing non-immunologic components. This treatment depletes the immune system existing in the blood at that time.
Problems:
While CIDP responds to this treatment it must be done routinely to harness any sustained improvement and makes the patient susceptible to opportunistic infection.
REFERENCES
Back to the Top.
Please send questions, comments and suggestions to jabeaghan@davidson.edu
Back to my home page
Back to the Immunology home page
Davidson Biology
Davidson College