Terminal deoxynucleotidyl transferase (TdT) is a DNA polymerase that adds non-germline encoded (N) nucleotides to the junctions between gene segments during somatic recombination in lymphocytes. This enzyme is distinct from other DNA polymerases in that it can add nucleotides to the 3’ end of gene segments without the use of a template strand. Because TdT adds non-template encoded nucleotides to the junctions between gene segments, it contributes extensively to the junctional diversity of the third complementarity-determining region of lymphocyte receptors. Thus, TdT is important in the establishment of a combinatorially diverse lymphocyte repertoire.
Lymphocytes, also known as B cells and T cells, are the main effector cells involved in the adaptive immune response, which is the antigen-specific branch of the immune system. The cells of the adaptive immune response are able to respond to specific foreign antigens through binding patterns. The ability of an organism's lymphocytes to respond to a given number of various antigens is referred to as the lymphocyte repertoire. A large lymphocyte repertoire is desirable to enable organisms to fight off a wide variety of infectious agents.
Somatic recombination provides a mechanism whereby organisms can maximize their lymphocyte repertoire based on a limited amount of genetic information. Several gene segments must be put together to make a functional lymphocyte receptor, and each individual has many copies of each gene segment. During somatic recombination, one copy of each gene segment is selected to become part of the gene for the B or T cell receptor. Many combinations of gene segments are possible, creating combinatorial diversity in the lymphocyte repertoire. Additional diversity is created at the junctions between gene segments by the addition and deletion of random numbers of P- and N-nucleotides. P-nucleotides, or palindromic nucleotides, result from the random cleavage of hairpin ends between gene segments to generate palindromic sequences of single-stranded DNA. Some of the DNA on the ends of gene segments may be deleted as well.
TdT is key to increasing the diversity of the lymphocyte repertoire through the addition of N-nucleotides onto the ends of the newly formed P-nucleotides. TdT adds an average of two to five nucleotides per N region, and G:C pairs are added more often than A:T pairs (Mickelsen et al., 1999). However, the number and sequence of both P- and N-nucleotides that are added is unique for each junction.
Figure 1: Chime Image of TdT.
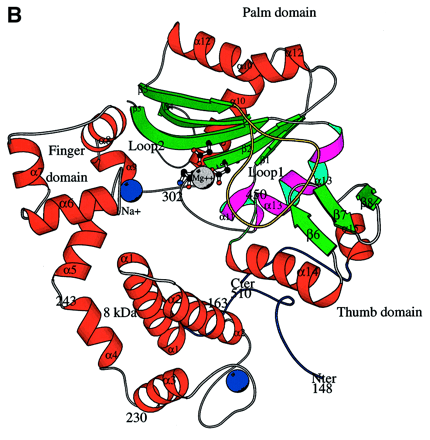
Delarue, et al. (2002) examined the structure of the catalytic core of murine TdT using crystallography. Their results indicate that TdT has a structure that is typical of other DNA polymerases, with the notable addition of a "lariat-like loop" that prevents the enzyme from interacting with a template strand. TdT falls into the polymerase family pol X, and it is more distantly related to most DNA polymerases including b, m and l. Unlike most DNA polymerases, TdT has little interaction with the bases it incorporates. Because of this, TdT can add a large number of nucleoside triphosphates to DNA, even those such as ribonucleotides that are not normally found in DNA. The enzyme contains a nuclear localization sequence to restrict its presence to the nucleus.

Figure 2: General Structure of TdT. Note the
"ring-like" shape of the enzyme.
Figure taken from Delarue,
et al. (2002) with permission.
Further examination of TdT showed that the catalytic site of the molecule contained two Co2+ ions, which is typical of the two metal ion mechanism of catalysis exhibited by all other known DNA polymerases. This result was also consistent with other studies that showed increased TdT activity in the presence of cobalt ions (Mickelsen et al., 1999). The main difference between TdT and other DNA polymerases seems to be the presence of a 16 amino acid loop that inhibits the enzyme's ability to interact with a template strand. This helps to explain why, in vivo, TdT usually adds dNTPs only to single-stranded "sticky" ends rather than blunt ends.
The overall structure of TdT showed the most homology with the closed conformational form of the DNA polymerase pol b. TdT seems to be sealed in a closed conformation, which is consistent with its ability to add dNTPs without first 'closing' onto a template strand (Delarue et al., 2002).
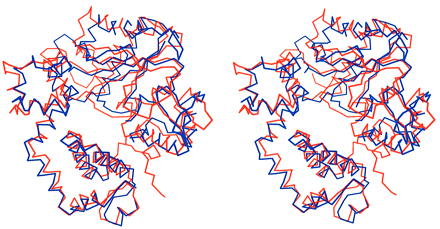
Figure 3: Comparison of TdT and pol beta structure.
Both enzymes are shown in a closed conformation. TdT (red) is superimposed onto
pol beta (blue).
Figure taken from Delarue,
et al. (2002) with permission.
TdT is found only in vertebrates, and its presence is correlated with the emergence of an adaptive immune system (Delarue et al., 2002). B and T cells express TdT early in their development as they undergo somatic recombination. Fetal lymphocytes express very low or no TdT.
B cells express TdT during the pro-B stage of development, as the heavy chain is being produced. N nucleotides are added to both the V-D junctions and the D-J junctions of immunoglobulin heavy chains. Once a functional heavy chain is made, the expression of TdT is down-regulated—N nucleotides contribute significantly to the diversity of the heavy chain, whereas few light chain rearrangements include the addition of N nucleotides. Using studies with transgenic and knockout mice, Wasserman, et al. (1997) determined that the production of the IgM heavy chain down-regulated the expression of TdT.
T cells express TdT throughout their double-negative and double-positive stages, until their receptors are either positively selected or die. Therefore, N nucleotides are added to all gene segment junctions in both a and b chains of T cell receptors. Cabaniols, et al. (2001) indicate that at least 90 percent of the junctional diversity of TCR chains comes from the addition of N nucleotides, as measured by sequencing TCRs of wild type and knockout mice.
TdT is dependent on many cellular factors that help to determine its expression level and function. One of these factors appears to be DNA-dependent protein kinase (DNA-PK). A study done by Mickelsen, et al. (1999) indicates that TdT "forms a stable complex with DNA-PK" that enables the enzyme to add N nucleotides to gene segment junctions. In individuals missing the regulatory subunit of DNA-PK, very few N nucleotides are observed. However, this study also shows that DNA-PK appears to control the activity of TdT in vitro by limiting the number and type of nucleotides added. DNA-PK is thought to interact with other cellular proteins to regulate TdT expression, although all the mechanisms are not known.
Essential to the control of every gene is its promoter. The TdT gene is unusual among eukaryotic genes in that its promoter does not contain a TATA box. Instead, an initiator element (Inr) appears to facilitate transcription of TdT. This initiator element is a sequence within the promoter that "directs accurate, basal transcription of TdT in both lymphoid and non-lymphoid cells" (Garraway 1996). The Inr also works in concert with an upstream sequence within the promoter called D' to enhance transcription in developing lymphocytes. The mechanisms whereby the Inr regulates TdT transcription seem to be similar to those typical of a TATA box. Garroway, Semple and Smale (1996) postulate that the TdT gene may utilize the Inr rather than a TATA box because other cellular factors are preferentially activated by the Inr.
Another aspect of TdT regulation involves the methylation of its promoter in mice. Nourrit et al. (1999) found that nonlymphoid organs had higher methylation levels in the Hha1 site of the TdT promoter than lymphoid organs such as the bone marrow and thymus. Because TdT only needs to be expressed in lymphocytes, other tissues can use methylation to prevent transcription of the gene. Methylation may also enable a cell to regulate the temporal expression of TdT; the thymus, for example, showed changes in its methylation profile throughout mouse development. (Note: mice only express TdT early after birth).
Studies using TdT -/- mice have shown that TdT is not necessary for a functional immune response, but that it increases the diversity of the lymphocyte repertoire. Knockout mice for TdT do not add any N nucleotides to the junctions between V(D)J gene segments (Benedict et al., 2000). A study by Tuaillon and Capra (2000) showed that knockout mice for TdT had a more limited repertoire than their wild-type counterparts; however, they responded competently to a variety of antigens. In addition, the gene segments in TdT -/- mice underwent more homology-mediated recombination than normal, indicating that TdT may inhibit this kind of recombination by its alteration of junction sequences. Lymphocytes produced by TdT knockouts are similar to those produced by fetal and neonatal mice—which normally do not express TdT (Benedict et al., 2000). Therefore, knockout individuals have a lymphocyte repertoire that would be typical of a fetus or neonate.
The overexpression of TdT also has observable consequences. If the enzyme is constitutively expressed, N nucleotides are added to Ig light chain V:J junctions (Tuaillon et al., 2000). When TdT is expressed prematurely in development, fetuses are unable to produce the clonal lymphocytes that they normally would, and the production of antibodies is impaired (Benedict et al., 2000). This result further illustrates the importance of TdT and its controlled regulation throughout development of cells and organisms.
TdT is commonly used by molecular biologists in the TUNEL assay (TdT-mediated dUTP digoxigenin nick end labeling) for detecting apoptotic cells. In this assay, TdT adds biotin-coupled uridine to the 3’ ends of DNA fragments that are formed during apoptosis. The biotin-labeled DNA fragments stain when exposed to enzyme-tagged streptavidin and a substrate, which becomes colored upon encountering the enzyme. Only cells that have undergone apoptosis are stained, so the number and position of apoptotic cells within a sample can be observed (Janeway et al., 647).
Figure 4: Apoptosis Patterns in Xenopus using
the TUNEL Assay.
This figure shows the apoptosis patterning in normally developing Xenopus
laevis embryos.
Darkly stained cells are undergoing apoptosis.
Figure taken from Gautier,
et al. (1998) pending permission.
Benedict CL, Gilfillan S, Thai TH, Kearney JF. 2000. Terminal deoxynucleotidyl
transferase and repertoire development. Immunol Rev 175:150-7.
Cabaniols JP, Fazilleau N, Casrouge A, Kourilsky P, Kannellopoulos JM. 2001.
Most alpha/beta T cell receptor diversity is due to terminal deoxynucleotidyl
transferase. J Exp Med 194(9):1385-90.
Delarue M, Boule JB, Lescar J, Expert-Bezancon N, Jourdan N, Sukumar N, Rougeon
F, Papanicolaou C. 2002. Crystal structures of a template-independent DNA polymerase:
murine terminal deoxynucleotidyltransferase. EMBO J 21(3):427-439. http://emboj.oupjournals.org/cgi/content/full/21/3/427
Accessed 2003 16 Feb.
Feeney AJ, Lawson BR, Kono DH, Theofilopoulos AN. 2001. Terminal deoxynucleotidyl
transferase deficiency decreases autoimmune disease in MRL-Fas-lpr mice. J Immonol
167(6):3486-93.
Garraway IP, Semple K, Smale S. 1996. Transcription of the lymphocyte-specific
terminal deoxynucleotidyltransferase gene requires a specific core promoter
structure. Proc Natl Acad Sci USA 93(9):4336-41.
Gautier J, Hensey C. 1998. Programmed cell death during Xenopus development:
a spatio-temporal analysis. World Wide Web Dev Biol 203:36-48. http://www.xenbase.org/xmmr/Marker_pages/pcd/TUNEL.html.
Accessed 2003 19 Mar.
Janeway C, Travers P, Walport M, Shlomchik M. 2001. Immunobiology: The Immune
System in Health and Disease. NY: Garland Publishing, 2001.
Mickelsen S, Snyder C, Trujillo K, Bogue M, Roth DB, Meek, K. 1999. Modulation
of terminal deoxynucleotidyltransferase activity by the DNA-dependent protein
kinase. J Immunol 163(2):834-43.
Nourrit F, Coquilleau I, D'Andon MF, Rougeon F, Doyen N. 1999. Methylation of
the promoter region may be involved in tissue-specific expression of the mouse
terminal deoxynucleotidyl transferase. J Mol Biol 292(2):217-27.
Tuaillon N, Capra JD. 2000. Evidence that terminal deoxynucleotidyltransferase
expression plays a role in Ig heavy chain gene segment utilization. J Immunol
164(12):6387-6397.
Wasserman R, Li YS, Hardy RR. 1997. Down-regulation of terminal deoxynucleotidyl
transferase by Ig heavy chain in B lineage cells. J Immunol 158(3):1133-8.
This page was produced by Melissa Breedlove.
Send any comments to mebreedlove@davidson.edu
Melissa's Home Page
Immunology Home Page.
Davidson College Home Page.