This web page was produced as an assignment for an undergraduate course at Davidson College.
Allergic Asthma
Disclaimer:
This web-page is an informative survey on the immunological
mechanisms and effector cells involved in allergic asthma. If you are concerned
that you have asthma or suffer from asthmatic symptoms please consult your
physician.
Preface:
The following information provides a general synopsis of the
immunological pathology of asthma from effector ligands, cell-cell mediated
interactions, and effector cell functions to the classification and differentiation
of clinical symptoms. There is also a section that discusses current asthma
treatments and new potential targets for future immune-based asthma therapy.
General Overview:
Asthma can be generally considered a hyper-responsive airway disease involving
chronic inflammation of varying austerity arising from various (genetic and
environmental) etiology ((Hang et al., 2003), (King 1999)). Asthma is a multi-cellular
redundant and self-amplifying disease stemming from an individuals hyper-response
to innocuous environmental antigens. The pathophysiology of asthma includes
mucus hyper-secretion, bronchial hyper-responsiveness, smooth muscle hypertrophy
(Schmitz et al., 2003 ), and airway obstruction (King 1999)
(Bronchial hyper-responsiveness is measure y a bronchial inhalation challenge
test where a patient is stimulated with metacholine or histamine (King 1999)).
Sometimes, chronic inflammation of the lungs is persistent even in the absence
of innocuous antigens and asthmatics can have hypersensitive airways to other
environmental antigens including viral and some bacterial infections. Inflammation
causes patients to suffer from some if not all of the following clinical symptoms:
chest tightness, wheezing, breathlessness, and coughing. (King 1999)
On a cellular level these asthmatic symptoms arise from the activation of
sub-mucosal mast cells by innocuous antigens (allergens) in the lower airways
resulting in mucous and fluid accumulation subsequently followed by bronchial
constriction. (Berin 2002) The immune response to asthmatic allergens is mediated
by CD4+ T helper 2 (Th2) cells, eosinophils, neutrophils, macrophages, and
IgE antibodies. Not surprisingly, these effector cells release cytokines that
also affect expression of adhesion molecules on epithelial cells (Janeway
et. al., 2001). Without effective treatment, proimflammatory cells in a dysregulated
asthmatics immune response initiate remodeling of airway tissues, commonly
called subbasement membrane fibrosis. For patients with severe cases, there
is a higher frequency of structural remodeling of the small airway matrix
compared to patients with less severe cases; however, the later are not precluded
from structural remodeling of the small airway matrix. This data emphasizes
the importance of treatment in both severe and mild asthma cases. (King 1999)
Clinical Phenotypes:
Asthma phenotypes are differentiated based upon the development of symptoms
and the severity of asthmatic lung inflammation. Asthma symptoms are typically
manifested at certain stages in life and can be classified into three general
categories: childhood asthma, late-onset asthma and occupational asthma. Childhood
asthma can arise from several different factors. Typically, a covirial infection
such as the rhinovirus, a family history of allergy, or atopy can result in
the development of childhood asthma. In childhood asthma, atopy usually results
from innocuous substances such as dust mites, pet dander, and fungi. Late
onset and occupational asthma exhibit different characteristics from childhood
asthma and probably have a different etiology. Asthma’s causation in
these circumstances may arise from constant exposure to environmental innocuous
antigens. The current distinction between late-onset asthma and occupational
asthma is merely the fact that the latter happens usually because of specific
antigen exposure related to work. It should be noted that 30-50% atopic individuals
do not develop asthma supporting the causation hypothesis that asthma is polygenic
and environment related.
Asthmatic inflammation is differentiated into three broad categories: acute,
subacute and chronic. Acute asthmatic inflammation involves the early recruitment
of cells in to the airway, while subacute asthmatic inflammation is characterized
by the activation of recruited and residual effector cells resulting in incessant
inflammation. Chronic asthma is defined by constant inflammation leading to
cellular damage, which in turn activates cellular repair. (King 1999)
Susceptibility:
Clinical identification of various aberrant asthmatic phenotypes and family
histories of asthma indicate a genetic predisposition to asthma development.
Moreover, population studies have recently correlated various genetic and
phenotypic polymorphisms to the onset and development of asthma.
Individuals with a genetic predisposition to asthma are designated as atopic
and have a robust response to allergens due to increased levels of IgE antibodies
(Ab) (Berin 2002). Clinical studies in Germany by Illi et al. corrlelated
atopic sensitization in atopic children with childhood asthma at age 7 compared
to children with out childhood asthma. Children in the study were considered
more susceptible to developing asthma by the age of seven if they had a family
history of asthma or atopy. This led the authors to concluded that a fundamental
factor relating asthma and maternal transmission may lead to different degrees
of sensitization and asthma phenotypes (Care must be taken when reviewing
these findings since approximately 1/3 of atopic children developed asthma).
(Illi et al., 1999)
At the molecular level, linkage analysis revealed that chromosomal regions
2q33, 5p15, 11p15, 17q11.1-q11.2, 19q13, and 21q21 (Hang et al., 2003) are
coupled to asthma and several polymorphic markers have been identified at
chromosomal region 14q (Kusser et al., 2001). PCR analysis of TAP1 ( Transporters
associated with antigen processing (TAP1) is a protein heterodimer responsible
from translocation of pepetides to MHC I (major histocompatibility complex)
surface glycoprotein. TAP is found in the chromosomal regions DQB1 and DRB1.)
gene polymorphism for 43 healthy people and 116 asthmatics revealed that TAP1
Acc1 allele polymorphism correlates to atopic bronchial asthma. Couple these
results with the fact that TAP1 may also activate intercellular adhesion molecule-1
(ICAM-1) and IFN-y responsiveness, TAP1 may be a potential genetic marker
for asthma. However Hang et al. point out that there results might also be
due to direct causation, natural selection, population stratification, statistical
artifact, and linkage disequilibrium. (Hang et al., 2003)
Another study by Kusser et al, showed a significant correlation between the
inheritance of the exon 1 allele BE1-2G for the B2 bradykinin receptor gene
and late-onset asthma among 77 children with asthma and 73 controls. Previous
studies showed that increased levels of bradykinin in asthmatics and stimulation
with bradykinin could induce bronchial constriction in asthmatics. Out of
three common alleles for B2 receptor gene: 2G, 3G, and 3T, the 2G allele had
the highest transcription rate while the 3T had the lowest transcription rate
suggesting transcriptional regulation of the B2 receptor gene may lead to
the development of asthma. (Kusser et al., 2001) However, this hypothesis
warrants further investigation due to a limited population sampling and demographics.
Moreover, asthma is a complex, polygenic disease influenced by numerous environmental
factors and there could be more than one genetic marker or causation.
Molecular Pathology:
A discussion of the molecular mechanisms underlying immune system activation
for allergen induced asthma includes several critical components of the immune
response: immune related receptors, effector ligands, functional roles of
effector T cells, and costimulatory molecules. Generally, allergen exposure
stimulates a Cd4+ Th2 immune response and the subsequent production of IgE
Ab. Re-exposure to allergen results in the recruitment of mast cells (via
high affinity IgE Fce receptors), eosinophils and other leukocytes. In particular,
mast cells that release the vasoactive amine, histamine, and other ligands
from large granules produce a local systemic hypersensitivity reaction (Janeway
et al., 2001). The ensuing inflammation amplifies an individual’s hypersensitivity
reaction by the recruitment of other cells and perpetuates the clinical symptoms
(wheezing, shortness of breath, and chest tightness) (King 1999).
Receptors:
There are three receptors linked to asthmatic pathogenesis: IL-1 receptpr
1 (IL-1R1), the Toll-like Receptor (TLR) and CCR8. The cytokine IL-1 promotes
the proliferation of Th2 cells and specific antibody responses. IL-1 also
induces the expression of exotaxin, an eosinophil chemoattractant, on pulmonary
epithelia cells. Schmitz et al. showed that, post allergen exposure, null
IL-1R1 mice had reduced CD4+ T cell proliferation relative to wild type controls.
Il-1R-/- mice also had limited pulmonary antibody responses, eosinophillia,
and globlet cell mucus production due to impaired CD4+ Th2 function and development.
These results, however, are for a mild asthma model and a more severe asthmatic
model with IL-R1null mutations did not alter immune responses. The authors
hypothesize, with data from concurrent studies, that IL-1 may play a role
in T-cell priming through induction of CD40L and OX40 receptor. (Schmitz et
al., 2003)
Another potential receptor involved in asthma responses is the Toll-like Receptor
(TLR). There are currently 10 known isoforms (TLR 1-10). The reactivity and
activation of TLR’s with host proteins suggests a functional role in
inflammatory states and in autoimmune responses. For example, the “hygiene
hypothesis” proposes that fewer bacterial infections in industrialized
and modernized nations is inversely proportional to the incidence of allergic
diseases. Since bacterial infections mount T helper cell 1(Th1) responses
through various TLRs it is possible that a reduction in TLR ligand on pathogens
coupled to allergen exposure means a reduction in shifts from a Th2 to Th1
immune response for hypersensitivity reactions. This also suggests that activating
TLR receptors in conjunction with allergen may be necessary to induce immune
deviation. (Heine et al., 2003)
Th2 and Th1 Cd4+ cells show differential receptor expression indicative of
the different effector and recruitment mechanisms for the respective cells.
Knockout mice for the CCR8 Th2 receptor showed deficient development in allergic
airway responses. These mice had reduced eosinophilia and cytokine production.
However, the bronchial hyperresponsivness was not reduced. Interestingly,
the correlation between these results and deficient CCR8 remains to be determined.
(Berin 2002)
Effector Molecules:
Cytokines and chemokines play a critical role in the development and recuirtment
of effector cells, respecitively. Asthma is typically characterized by IL-4,
Il-5 and Il-13 cytokine production for IgE, eosinophils and mucus (Schmitz
et al., 2003). Specifically, IL-4 and IL-13 induce isotype switching and IgE
production, while IL-5 exherts regulatory activity on eosinophil growth, differentiation
and activation (Berin 2002). Other notable cytokine expression in the asthmatic
lung includes granulocyte macrophage-colony-stimulating factor mRNA. Dissimilarly,
local concentrations of INF-gamma do not increase in the lung. The presence
and expression of the aforementioned cytokines suggests a CD4+ Th2 cell immune
response to asthma. (Berin 2002)
Additional studies have shown that pro-inflammatory cytokines IL-1B and IL-6
alter asthmatics airways. In status asthmaticus (acute respiratory failure)
there are substantial increases in IL-1, IL-6 and TNF-alpha. These cytokines
can have both immune and non immune related functions. Most notably, IL-1
alpha or IL-1 beta can induce loss of appetite, acute phase protein production,
increases in adhesion molecules, vasodialation, increased hematopoesis, fever,
growth factors, and release of matrix metalloproteinases. (Schmitz et al.,
2003)
Chemokines are produced by a variety of cells and yet only eotaxin, a CC chemokine
that acts on eosinophils, correlates with asthmatic symptoms (Berin 2002).
Animal studies suggest, however, that chemokines play a combitorial role in
the development of asthma by acting on the same cells. Neutralization of MIP-1alpha,
eotakin, MCP-5 or MCP-1 or inhibited RANTES in murine models decreased bronchial
hperresonsivness after ovalbumin challenge. Different receptors on Th1 and
Th2 cells suggest different recruitment mechanisms for different immune responses.
For example, Th2 cells preferentially express CCR8 and CCR4. TARC, a ligand
for the foregoing Th2 receptors, is upregulated in bronchial epithelial cells
in allergen challegened astmatics. Figure 1 outlines a proposed mechanism
for TARC in the chemotaxis of Th2 cells during allergen simulation. Therefore,
TARC may also represent a new protein involved in allergen induced asthma
and a potential therapeutic target for neutralization or small molecule inhibition.
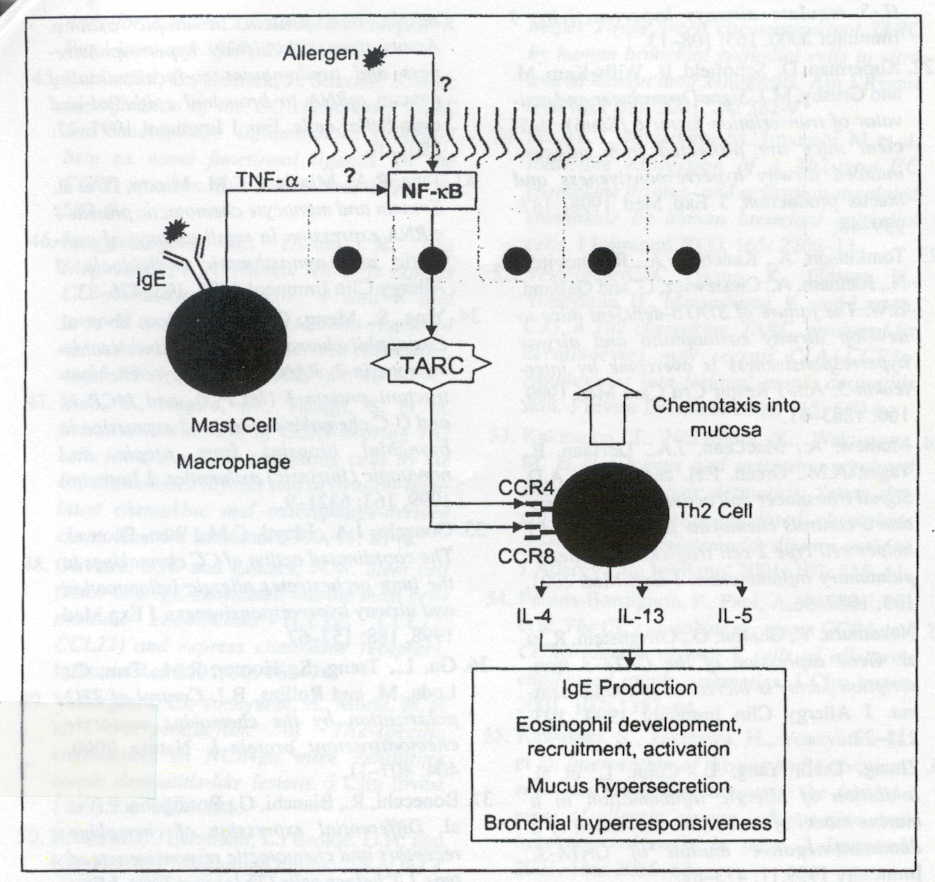
Figure 1: Production of IL-1 and TNF-alpha leads to the induction of the transcription factor NF-kB and TARC expression. TARC expression leads to the recruitment of Th2 cells (Berin 2002). Premission pending for image use.
These effector ligands also up regulate a distinct set of transcriptions factors. In particular, there are two transcription factors that have a predominant role in asthma hypersensitivity reactions. Altering the concentrations or effectiveness of these transcription factors illustrates the importance of effector molecules in mediating a hypersensitivity reaction. One of these transcription factor is Stat-6 and is involved in the signal transduction pathway affected by IL-4, IL-5 and IL-13. It is believed that IL-4 and IL-13 are necessary to induce Stat-6, and in turn Stat-6 activates genes responsible for bronchial hyper-responsiveness and allergic inflammation. Stat-6 is also vital for the development of CD4+ Th2 cells and Stat-6 null mutants fail to induce asthma due to a lack of IL-5 production. Adoptive transfer of Stat 6 +/+ T cells into Stat 6 null mutants also demonstrated that Stat 6 is essential for eosinophilia mucus production. The second important transcription factor in allergic asthma is GATA 3, which is involved in Th0 to Th2 differentiation and induced in the asthmatics airways. (Berin 2002)
Costimulatory Molecules:
Several costimulatory factors are believed to play a crucial role in the development
of tolerance and immunity. One set of costimulatory molecules is OX40 and
OX40L, members of the tumor necrosis factor family of receptors. In null mutant
mice for OX40, large numbers of recruited eosinophils, IgE production and
concentration of Th2 cytokines in the serum and bronchalveolar lavage were
dependent upon the presence of OX40. Moreover, globlet cell hyperplasia, mucus
production, and airway hyperresponsiveness (AHR) were suppressed in the absence
of OX40, illustrating the importance of OX40 in Th2 asthma responses.
Another important costimulatory set of molecules is ICOS and ICOSL (Note:
ICOS is expressed on T cells and ICOSL is expressed on dendritic cells). Its
importance in the immune response is highlighted by the fact that blocking
ICOS-ICOSL interactions inhibits respiratory tolerance and suppresses regulatory
T (Tr) cells development. Inhibition of ICOS during initial stimulation and
differentiation of naive T cells results in the production of more Th1 cells
suggesting smaller concentrations of Th2 developmental cytokines. Murine models
deficient in ICOS have decreased IgE production, Th2 cytokines, and (AHR).
ICOS null mutations in a murine model suppress IL-4 and IL-13 production but
make the mice susceptible to inflammatory lung disease by airway challenge
in primed mice. This result and additional data led Akbari et al. to hypothesize
that a Th2 immune response to allergens may abate Tr cell counts due to diminished
IL-10 production. If this hypothesis is correct it represents a new possibly
avenue for therapy: IL-10 induced tolerance. (Akbari et al. 2003)
T cells:
The exact mechanism, factors and nature of an allergic asthma immune response
are still being defined. It is believed that symptoms are manifested because
of a Th2 mediated immune response. Figure 2 provides a schematic representation
of the ligands involved in the regulation, recruitment, and effector functions
of Th2 cells (Larche et al., 2003). However, other T cells may play a role
in allergic asthma. Zuany-Amorim et al. observed that pulmonary allergic inflammation
in mice with out gamma-delta T cells lead to decreases in specific IgE, IgG1,
pulmonary IL-5 concentration, and eosinophil and T cell recruitment relative
to wild type mice. The authors concluded that gamma-delta T cells are crucial
for IL-4 dependent IgE and IgG1, and Th2 cell-mediated pulmonary inflammation.
(Zuany-Amorin et al., 1998) Data for the importance of human gamma-delta T-cells
is still being debated (Larche et al., 2003). Futhermore, there is evidence
that CD8+ cytotoxic T-cells, in particular, cytotoxic type 2 lymphocytes,
have a role in the asthma process. For example, in a murine model virus-specific
CD8+ T cells switched to IL-5 production and caused airway eosinophilia, suggesting
that pathogens or chemical haptens can modify antigens
thereby inducing CD8+ cytotoxic T cell responses. (Larche et al., 2003) Lastly,
there has been a report that IL-10 secreting Tr cells prevent AHR development
in allergen sensitized mice, indirectly suggesting that wild type responses
to allergens involve Tr mediated immune suppression. (Akbari et al., 2003)
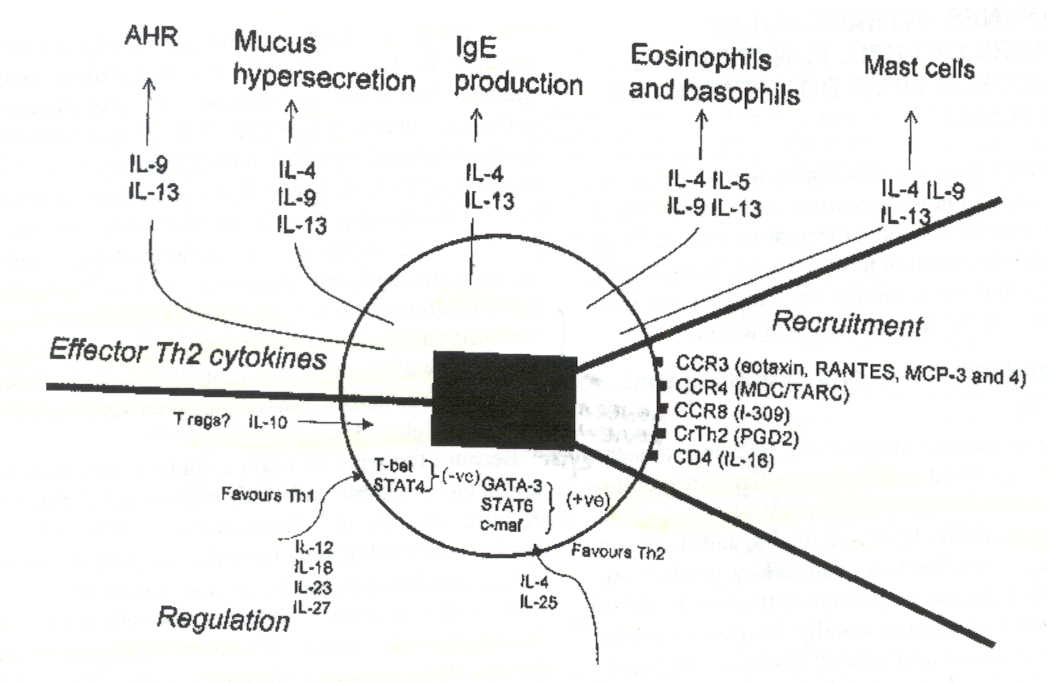
Figure 2: Schematic representation of the ligands secreted and effected by
Th2 cells during an allergic asthma. The numerous ligands suggest particular
cytokine neutralization and injection strategies to minimize allergen induced
asthmatic symptoms (Larche et al. 2003). Premission pending for image use.
Immunotherapy / Treatments:
Since allergic asthma constitutes both genetic and environmental components
there are numerous possibilities for developing new treatments for asthmatics.
To minimize the manifestation of allergic symptoms due to environmental stimulus,
patients could simply avoid allergens, however, this is not pragmatic and
exposure to allergens is often unavoidable. Neutralizing the genetic component
of asthma may be more difficult, simply because of the polygenic nature of
the disease. However, either treatment of the symptoms or altering the immune
response can often lead to minimizing asthmatic symptoms. Current treatments
rely on aersol inhalers, anti-histamine treatments, and regiments of corticosteroids
(Larche et al., 2003) to diminish aberrant symptoms. Several studies showed
that steroids reduce T-cell activity. (Larche et al., 2003) However, prolonged
steroid use exacerbates unwanted side effects and Woisetschlager et al. suggest
altering the immune response to asthma by direct or indirect abrogation of
IgE Ab. Figure 3 outlines how decreases in IgE affect other immune related
cells. New potential therapeutics would ideally minimize the “direct”
functionality of IgE by neutralizing IgE, for example with mAb rhuMab-E25
or neturalizing the family of IgE receptors. Indirect abrogation of IgE would
involve developing therapeutics to inhibit isotype switching and transcription.
Additionally, the potential for therapeutics designed to alter cell-cell interactions
could mitigate asthmatic symptoms. Furthermore, Woisetschlager et al. suggest
that curtailing isotype switching would target two key mechanisms, first,
the activation of IgE germline gene transcription and, second, DNA switch
recombination. Since Stat 6 is a critical transcription factor in germline
gene activation Woisetschlager et al. propose that a possible inhibitor of
Stat 6 would mitigate IgE production and have a direct effect on B lymphocytes.
This inhibitor could also reduce Th2 cell differentiation since Stat-6 is
necessary for naive T-cell differentation. One potential inhibitor is the
(S)-(+) enantiomer 4-(1-phenylethylamino)quinazoline which works at uM concentrations
by inhibiting IgE germline transcription. Small molecules inhibitors could
also bind to promoter regions to deactivate transcription. One such class
of molecules, 2-aminoetoxy-modified poly pyrimidine oligonucleotides formed
a triplex DNA structure that inhibited transcription factor binding for the
promoter region of IgE. Another target of IgE production was the protein SWAP-70,
which is B-cell specific and involved in recombination. SWAP 70 -/- mice had
defective IgE immune responses while other isotypes were not as severely affected.
Another therapeutic agent in clinical development is a mAb to CD23 or FceRII,
a low affinity IgE receptor on B cells. (Woisetschlager et al. 2002) There
are many possibilities for new asthma therapies but the foregoing therapies
merely represent ideas on paper that will not necessary show positive results
at the clinical bedside. Additionally, some of these therapies my elicit unwanted
side effects or leave individuals susceptible to other pathogens.
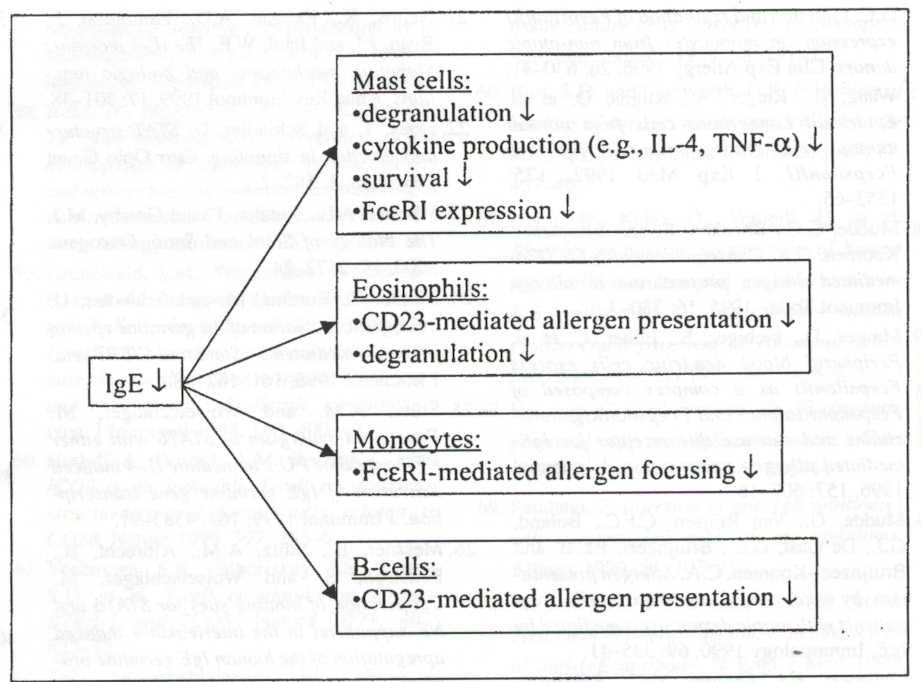
Figure 3: Reducing IgE concentration results in: ihibition of mast cell and eosinophil degranulation and cyokine production, decreased mast cell counts, decreased IgE receptor expression, and reduced Cd23 expression, and/ or new Th2 cells from CD23 presentation of allergen (Woisetschlager et al. 2002). Premission pending for image use.
Conclusion:
The dichotomous nature of asthma, simulated by both environmental innocuous
antigen and an atypical immune response makes it an interesting and problematic
disease to study. Numerous factors could be the causation of the disease and
it is up to future research both at the molecular and clinical levels to find
the critical mediators of allergen induced hypersensitivity reactions to make
new and more effective treatments. Ultimately, the best treatments will probably
aim at immune deviation, switching the allergic asthma response from Th2 cells
to Th1 cells
References:
Janeway C. A., Travers P., Walport M., Shlomchik M. J. 2001. Immunobiology. New York, NY: Garland Publishing. p. 683-707, 486-487, 477-478.
Hang L-W, Hsia T-C, Chen W-C, Chen H-y, Tsai F-J. 2003. Tap1 Gene Acc1Polymorphism is Associated with Atopic Bronchial Asthma. Journal of Clinical Laboratory Analysis. 17: 57-60
King T. E. A New Look at the Pathophysiology of Asthma. 1999. Journal of the National Medical Association. 91 (8): 9S-15S.
Berin M.C. 2002. The Role of TARC in the Pathogenesis of Allergic Asthma. Drug News Perspect. 15(1): 10-16.
Schmitz N, Kurrer M, Kopf M. 2003. The Il-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur. J. Immunol. 33: 991-1000.
Illi S, Mutius E v, Lau S, Nickel R, Niggemann B, Sommerfeld C, Wahn U. 2001. The pattern of atopic sensitization is associated with the development of asthma in childhood. J Allergy Clin. Immunol. 108 (5): 709-714.
Kusser B, Braun A, Praun M, Illi S, Mutius E v, Roscher A. 2001. Polymorphisms in the Bradykinin B2 Receptor Gene and Childhood Asthma. Biol. Chem. 382: 885-889.
Heine H, Lien E. 2003. Toll-Like Receptors and their Function in Innate and Adaptive Immunity. Int Arch Allergy Immunol. 130: 180-192.
Akbari O, Stock P, DeKruygg R H, Umetsu D T. 2003. Mucosal Tolerance and Immunity Regulating the Development of Allergic Disease and Asthma. Int Arch Allergy Immunol 130: 108-118.
Larche M, Robinson D S, Kay B. 2003. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 111 (3): 450-463
Zuany-Amorin C, Ruffie C, Haile S, Vargaftig B B, Pereira P, Pretolani M. 1998. Requirement for gamma delta T Cells in Allergic Airway Inflammation. 280: 1265-1267.
Woisetschlager M, Stutz A M, Ettmayer P. 2002. Prevention of Immunoglobulin E Production as a Therapeutic Target. Drg News Prespect. 15 (2): 78-84.
Alan Cubre's Immunology Home Page
Davidson College Biology Department
© Copyright 2002 Department of Biology, Davidson College, Davidson, NC 28035
Send comments, questions, and suggestions to: alcubre@davidson.edu