"This web page was produced as an assignment for an undergraduate course at Davidson College."
Immune Related Disease: Malaria
Disease Pathogenesis
Malaria affects approximately 300 million people every year and results in 1 million deaths wordwide (WHO, 2003). It is caused by infection of one of four species of intracellular protozoan parasites of the genus Plasmodium, P. falciparum, P. vivax, P. ovale, or P. malariae (Robbins, 1999). P. falciparum causes the most severe symptoms including severe anemia, cerebral symptoms, renal failure, pulmonary edema, and death and thus is the focus of most research and this webpage (Robbins, 1999). Whenver Plasmodium is stated on this page it refers to P. falciparum. P. falciparum is the most severe because this strain of parasite can invade red blood cells (RBC) of any age while the other strains can only infect either young or old red blood cells. The plasmodium matures in the gut of the female Anopheles mosquito and is passed from the mosquito to humans through the saliva when the insect bites (http://www-micro.msb.le.ac.uk/224/Malaria.html).
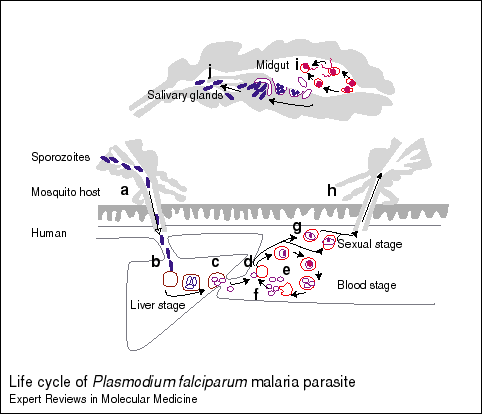
Plasmodium Life-cycle in Anopheles Mosquito: The male and female sexual forms of merozoites are fertilized in the mosquito midgut and form zygotes. They differentiate into ookinetes and migrate through the gut wall and divide into sporozoites in oocysts in the mosquito gut wall. These sporozoites migrate into the mosquito saliva and are injected into the blood stream (peripheral circulation) when an Anopheles mosquito bites a human (http://www-ermm.cbcu.cam.ac.uk/dcn/fig001dcn.pdf).
Plasmodium Life-cycle in Humans: Within a few minutes, the injected sporozoites invade the hepatocyte cells in the liver and after about one week they multiply into thousands of merozoites by asexual multiplication (http://www-ermm.cbcu.cam.ac.uk/dcn/fig001dcn.pdf). The intermediate stage is called a trophozoite (Langhorne, 1998). This large number causes the hepatocyte to rupture and release the merozoites into the blood stream. The merozoites next infect red blood cells (RBC) and multiply asexually within 48-72 hours increasing their numbers by about 20x. As seen with the hepatocytes, this large number of merozoites causes the RBC to rupture and the released merozoites invade more RBC. A cycle results that is thought to be the cause of the periodic fevers of malaria. Some merozoites differentiate into male and female forms and if the infected individual is bitten by an Anophelesmosquito, the cycle will continue as the mosquito will be infected by the male and female merozoites (http://www-ermm.cbcu.cam.ac.uk/dcn/fig001dcn.pdf).

Figure 1. Life-cycle of P. falciparum. General dipiction of Plasmodium life-cycle in mosquito and man. Permission pending from Daniel Carucci et al http://www-ermm.cbcu.cam.ac.uk/dcn/fig001dcn.htm. Click on the link to see their description of the different stages that correspond to the letters in the figure. I have summarized this information above.
The next figure is a more detailed depiction of the different stages of the Plasmodium life-cycle:
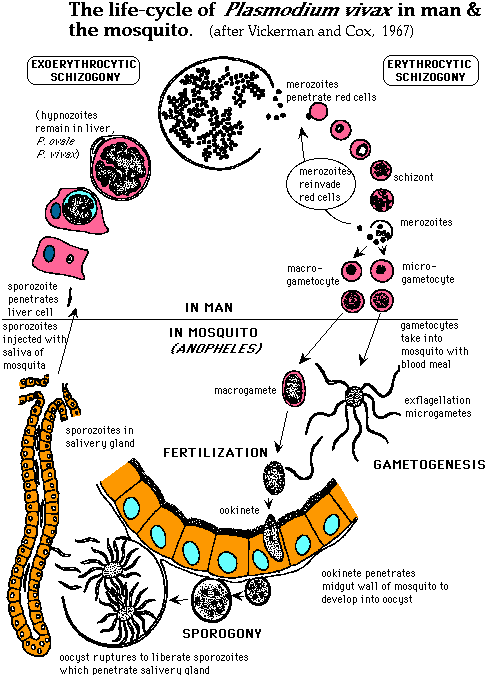
Figure 2. Life-cycle of P.vivax. Although this figure depicts the life-cycle of P. vivax, the steps depicted are the same for P. falciparum and are displayed in more detail than in any figures I found for P. falciparum. The main difference is that P.vivax hypnozoites can remain in the liver cells while P. falciparum hypnozoites do not. Permission pending from Dr. Alan Cann http://www-micro.msb.le.ac.uk/224/Malaria.html.
Host Immune Reponse
Complex Reponse: The Plasmodium exists in humans in two extracellular forms, sporozoites and merozoites, and in intracellular stages in hepatocytes and erythrocytes. The Plasmodium parasite stimulates CD4 and CD8 T cells, gamma-delta T cells, macrophages, natural killer (NK) cells, and B cells (Langhorne, 1998). Since the malaria parasite, P. falciparum lives inside of human cells for much of its life cycle, it is able to avoid macrophages and antibodies (Langhorne, 1998). Macrophages and antibodies are able to engulf and bind to the parasite during the sporozoite and merozoite stages in the blood stream, but these stages are transient and the parasite exists in large enough numbers that some will infect host cells before being destroyed by the host's immune system (Rouzine, 2003). The key to survival of the malaria parasite in the host seems to be its ability to synchronize its release from the host's cells, the hepatocytes and erythrocytes. Inflammatory cytokines are released in response to initial infection with sporozoites, but as stated previously, some sporozoites will survive to infect hepatocytes. The host immune system is tricked into thinking that it has removed all of the pathogen, but in reality the parasite is just replicating inside the host cell (Rouzine, 2003). After replication in the hepatocytes, when the large number of merozoites causes the cell to burst, the host immune system must induce inflammatory cytokines and an antibody response. But, since both of these responses take time, many merozoites have already infected erythrocytes before the host can mount an immune reponse (Rouzine, 2003). But, also an acquired antibody response takes days to weeks to develop and the replication cycle is usually on the order of 3 days (Rouzine, 2003).
Lack of MHC presentation: All nucleated cells in the body express MHC class I molecules, but hematopoietic cells express them most highly. Only antigen presenting cells express MHC class II molecules normally, but other cell types can if they are exposed to interferon-gamma (Janeway, 2001). P. falciparum invades hepatocytes, which express very low levels of MHC class I molecules and red blood cells, non-nucleated cells which express little or no MHC class I molecules (Janeway, 2001).
Humoral Response: Most individuals exposed to the malaria parasite, Plasmodium, make antibodies against epitopes on the surface of the sporozoite, but the immune response only partially removes the parasite and individuals are prone to repeat infections (Krogstad, 1996). B cells make antibodies to the surface antigens of sporozoites and have been shown to prevent the invasion of hepatocytes, but the problem is the large number of sporozoites. In order to effectively clear the sporozoites, a higher concentrations of antibodies is needed (Langhorne, 1998). The antibody response is very important in controlling the primary infection, especially the blood stage parasites (sporozoites and merozoites) and a large increase in production of IgG antibodies specific to sporozoites occurs following infection (Langhorne, 1998). The binding of antibody to the parasite or to infected cells leads to their elimination through various mechanisms. Antibodies to surface proteins of merozoites or surface molecules of infected erythrocytes leads to clustering and lysis by complement activation or phagocytosis which either blocks the infection of red blood cells or kills the infected RBC (Langhorne, 1998). In areas where malaria is endemic, as individuals are repeatedly infected, they develop antibodies to the surface proteins of sporozoites and over time, they may be able to have enough antibodies present prior to infection and in high enough concentrations to prevent infection of hepatocytes (Langhorne, 1998). Therefore, non-immune individuals traveling to areas with endemic malaria are more vulnerable to infection that the inhabitants of the area.
T-cell Mediated Response: There is no CD8 T cell response for the erythrocytic stage because RBC's do no express MHC class I or II molecules. The CD8 T cell reponse is activated by the hepatocytic stage, but the level of activation is low and the RBC stage still escapes from the CD8 T cell reponse (Torre, 2002). In rats, merozoites have been observed to suppress the low level CD8 T cell response that was initiated by infected hepatocyte cells (Ocana-Morgner, 2003). Merozoites interact with dendritic cells, inhibiting their maturation and capacity to initiate immune responses by inverting the interleukin IL-12/IL-10 secretion pattern. The interaction of blood stage parasites with dendritic cells induces the secretion of soluble factors that inhibit the activation of CD8+ T cells and suppress protective CD8+ T cell responses against the hepatocyte stage (Ocana-Morgner, 2003). CD4 T cells are activated in response to the erythrocytic stage of infection with Th1 cells activated during acute infection and Th2 cells activated in chronic infection (Langhorne, 1998). Merozoite surface protein 1 (MSP-1) has been documented as one of the targets of cellular and humoral immune responses in humans (Burns, 2003). Exposure to MSP-1 induced high levels of antigen-specific Th1 (IFN gamma) and Th2 (IL-4 and IL-10). One reason for the proposed poor cell-mediated response to sporozoites is that the reponse is restricted by the individuals HLA haplotype (Torre, 2002).
Humans vaccinated with irradiated sporozoites protects against sporozoite challenge and is mediated by CD8 and CD4 T cells and also maybe gamma-delta T cells (Langhorne, 1998). The T cells apear to induce the death of infected hepatocyte cells via release of nitric oxide (NO) and interferon gamma (IFN gamma). CD8 T cells that recognize sporozoite and liver-stage antigens have been identified in the blood of immunized humans and in lymphoid organs of rodents (Langhorne, 1998).
Innate Immune Reponse: RBC infected with Plasmodium induce the innate immune response involving inflammatory cytokines that slow the growth of early blood-stage infections(Robbins, 1999). Macrophages activated by IFN gamma and tumor necrosis factor alpha (TNF alpha) can kill the merozoites inside the RBC through NO and oxygen radicals. Although an inflammatory response can control the infection, mice without IFN gamma, TNF alpha, and NO are still able to control and remove malaria parasites (Langhorne, 1998).
Pathogenesis from the Immune Response Itself: Hypoglycemia, anemia, and cerebral malaria are thought to be the result of dysregulated cytokine response (Langhorne, 1998). Toxins released by the malaria parasite induce macrophages to produce cytokines such as TNF alpha, which has been observed to worsen cases of cerebral malaria. Also, CD4 T cells and gamma delta T cells produce IFN gamma which is thought to increase the inflammatory response and thus make the pathology of the disease worse (Langhorne, 1998).
Strategies for Evasion: The large number of parasites produced through replication compensates for losses of cells due to death from the immune response (Krogstad, 1996). The malaria parasite sheds the surface coat of sporozoites when bound with antibodies, through evolution, has selected peptide sequences the are not presented well by MHC alleles, and many of its proteins are antigenically diverse (Langhorne, 1998). During the parasites life-cycle, it changes so much that antibodies against sporozoites are ineffective against the merozoites and those effective against the merozoites are ineffective against the sexual stages (Krogstad, 1996).
One of the unanswered questions is how plasmodia repetitively infect individuals without stimulating an effective immune resopnse, because acquired immunity does not prevent reinfection but does reduce the severity of disease (Krogstad, 1996).
Clinical Symptoms
Recurrent fever and chills at irregular intervals is a notable symptom that result from the synchronous lysis of infected RBC (Krogstad, 1996). Headaches, general weakness, and anemia are also common symptoms of the disease (Robbins, 1999).
Clinical illness is caused by the erythrocytic stage of the parasite (Robbins, 1999). The first symptoms and signs of malaria are associated with the rupture of RBC's when merozoites mature and their release into the blood stream that triggers a host immune response (Robbins, 1999). The cytokines and reactive oxygen intermediates are probably responsible for the fever, chills, sweats, weakness, and other systemic symptoms associated with malaria (Robbins, 1999).
As RBC infected with Palciparum mature, they develop knobs which bind to molecules on endothelial cells such as thrombospondin, ICAM-1, and CD36 and block the blood flow in peripheral vessels (Krogstad, 1996). The sequestration of the infected RBC in the peripheral blood vessels causes microvascular disease that reduces the blood flow to the brain, lung, and kidney (Krogstad, 1996). The blockage of capillaries feeding the brain has been believed to be the cause of a coma (cerebral malaria), but hypoglycemia and cytokines also contribute to this complication (Krogstad, 1996). Hypoglycemia is thought to result from glucose consumption by the large number of parasites and decreased glycogen because the patient hasn't eaten because he or she hasn't been feeling well (Krosgstad, 1996). Renal failure and pulmonary edema can also result from malaria (Krogstad, 1996).
Diagnosis
Most often, malaria is diganosed by observation of the parasite in the blood through a thick or thin giemsa-stained blood smear (Krogstad, 1996). Fluorescent staining can also be done with acridine orage, DNA probes can be used in combination with blood smears, and the amount of antibody to Plasmodium in the blood can be measuerd (Krogstad, 1996).
Treatment
Chemoprophylaxis agents are used to kill the parasite after it has gained access to the body but before it causes the rupture of host RBCs, which causes the symptoms of malaria (Krogstad, 1996). This may be done by administering drugs that attack the parasite in either the liver or the blood stage, but most antimalarial drugs attack parasites in the blood stage (Krogstad, 1996).
Chloroquine phosphate, mefloquine (Lariam), and doxycycline are used for chemoprophylaxis and are taken by individuals traveling to areas in which malaria is a known problem (Krogstad, 1996). Unfortunately, many mosquitos have been resistant to chloroquine phosphate and mefloquine, so researchers are searching for alternate drugs that can be used over long periods (Krogstad, 1996). The disease itself is treated with chloroquine phosphate, quinine, and mefloquine and severe cases of cerebral malaria are treated with intravenous quinine (Krogstad, 1996). Blood glucose levels must be monitored during treatment because quinine stimulates the release of insulin from pancreatic beta cells and thus hypoglycemia is a common complication of treatment (Krogstad, 1996). Quinine and chloroquine function by binding to blood proteins and forming complexes which are toxic to the malarial parasite. The side effects of quinine include ringing in the ears, temporary deafness, blurred vision, nausea and upset stomach.
The merozoites replicating inside of RBC's ingest haemoglobin into food vacuoles and utilize exopeptidases and endopeptidases to break down haemoglobin into haemozoin which contains cytotoxic and lytic ferriprotoporphyrin IX (Fitch, 1983). A parasite protein, 'haembinder', sequesters ferriprotoporphyrin IX into the inert haemozoin complex and protects Plasmodium membranes from damage.
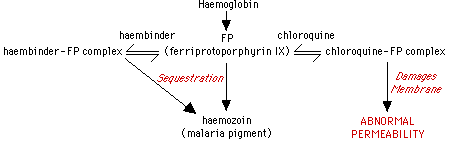
Figure 3. Flow chart showing the functional mechanism of chloroquine in killing the malaria parasite. Obtained from Dr. Fitch <"Mode of action of antimalarial drugs in: Malaria and the red cell." CIBA Foundation Symposium 94: 1983> with permission pending.
Chloroquine and quinine function to treat malaria because they work against parasites that degrade haemoglobin. Chloroquine prevents neutralization of the toxic ferriprotoporphyrin IX . The Plasmodium takes up Chloroquine into food vacuoles where it competes with the haembinder to form a compound with ferriprotoporphyrin IX (Fitch, 1983). Most of the ferriprotoporphyrin IX is sequestered into haemozoin instead of forming haembinder complexes and thus are able to lyse RBC infected with Plasmodium. Resistant parasites are unable to produce haemozoin, but they are still able to digest haemoglobin (Fitch, 1983). Quinine and mefloquine also cause blebbing of the parasite membranes and formation of haemozoin aggregates (Fitch, 1983). Mefloquine binds to normal and infected RBC membrane lipids. Mefloquine does not bind to hemoglobin, but does bind to ferriprotoporphyrin IX (Chevli, 1982).
Vaccination: Researchers are attempting to make vaccines that target antigens of sporozoites. They are also interested in making a vaccine to antigens of the gametes in order to prevent the transmission to the mosquito and protect the population from malaria (Langhorne, 1998). The most promising protein to use in a vaccination with adjuvant appears to be merozoite surface protein 1 (MSP-1) and Epp et al. have cloned and purified the protein and Graves et al. have begun initial clinical trials that have shown promising but not definative results (Epp, 2003; Graves, 2003).
References:
Burns JM, Flaherty PR, Romero MM, and Weidanz WP. "Immunization against Plasmodium chabaudi malaria using combined formulations of apical membrane antigen-1 and merozoite surface protein-1." (2003) Vaccine 21:1843-52.
Carucci, DJ, Gardner MJ, Tettelin H, Cummings LM, Smith HO, Fraser CM, Venter JC and SL Hoffman. "The malaria genome sequencing project: complete sequence of Plasmodium falciparum chromosome 2." Parassitologia. (1999). 41:69-75.
Chevli R and Fitch CD. "The antimalarial drug mefloquine binds to membrane phospholipids." (1982) Antimicrob Agents Chemother. 21:581-6.
Epp C, Kauth CW, Bujard H, and Lutz R.J. "Expression and purification of Plasmodium falciparum MSP-1(42): A malaria vaccine candidate." (2003) Chromatogr B Analyt Technol Biomed Life Sci, Mar 25; 786:61-72.
Fitch C. D. "Mode of action of antimalarial drugs in: Malaria and the red cell." (1983). CIBA Foundation Symposium 94.
Graves P and Gelband H."Vaccines for preventing malaria." (2003). Cochrane Database Syst Rev. 1.
Janeway, C., Travers, P. Walport, M., Shlomchik, M. Immunobiology: The Immune System in Health and Disease. New York, New York: Garland Publishing. 2001.
Langhorne, Jean and Anthony Holder. Malaria. Encyclopedia of Immunology. Volume 3. 2nd Edition. Peter Delves, Ed. San Diego: Academic Press. 1998.
Krogstad, Donald. Malaria: Diseases of Protozoa. Textbook of Medicine. Volume 2. 2oth Edition. Bennett, J. and Fred Plum, Ed. Philadelphia: W.B. Saunders Company. 1996.
Ocana-Morgner C, Mota MM, and Rodriguez A. "Malaria blood stage suppression of liver stage immunity by dendritic cells." (2003). J Exp Med 197:143-51.
Robbins, Ramzi S. Cotran, Vinay Kumar, Tucker Collins, Stanley L. Robbins and Bill Schmitt . Pathologic Basis of Disease. 6th edition. New York: W B Saunders. 1999.
Rouzine IM and McKenzie FE. "Link between immune response and parasite synchronization in malaria." (2003). Proc Natl Acad Sci (PNAS) 100:3473-8.
Torre D, Speranza F, Giola M, Matteelli A, Tambini R, and Biondi G. "Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria." (2002) Clin Diagn Lab Immuno 9:348-51.
World Health Organization (WHO). <http://www.who.int/en/> Accessed April 24, 2003.
The biology of malaria parasites: Report of a WHO Scientific Group. WHO Technical Report Series, 1987.
Send comments, questions, and suggestions to: sahenry@davidson.edu