This web page was produced as an assignment for an undergraduate course at Davidson College.
Poliovirus
Basics
Poliovirus is a human enterovirus, placing it in a family of viruses that reproduce in the gastrointestinal tract at the start of their infection. It typically infects young children, conferring mild flu-like symptoms in the majority of cases. A small percentage of polio cases result in paralysis or other more severe symptoms. Relatives of the poliovirus include other members of the large picarnovirus family, including foot and mouth disease virus, hepatitis A virus, rhinoviruses, and coxsackie viruses. Icosahedral in shape (having 20 triangular faces and 12 corners), poliovirus contains four virus-specific protein subunits (VP1, VP2, VP3, and VP4). Sixty copies of each of these arrange to give the small, non-enveloped virus its structure. Also contained within the virus is a 7500 nucleotide RNA genome with one open reading frame encoding a 220 kD polyprotein (Hogle et al., 1985).
Transmission and Route of
Infection
Poliovirus is typically acquired through ingestion of contaminated matter and enters tissues through our gastrointestinal mucosal surfaces. The virus targets cells bearing CD155, the receptor for the poliovirus, a gene only present in humans and old-world primates. Characteristic of enteroviruses, replication first happens in the throat and intestinal-associated tissues before entering the blood and lymph. Poliovirus can infect follicular-associated epithelial cells as well as cells in the Peyer’s patches and tonsils that lie under the epithelial surface. However, damage to epithelial cells is virtually nonexistent, while significant damage is seen in the tonsils and Peyer’s patches, suggesting that most viral replication takes place here. The microfold cells (M-cells), a part of the gastrointestinal epithelium, express CD155 on both their apical and basolateral surfaces. M-cells cells transport poliovirus directly from the gut to the lymphoid follicles of Peyer’s patches. Infection is aided by the fact that these epithelial cells have little IgA or mucus secretions, exempting the virus from one of the first lines of host defense (Iwasaki et al., 2002).
In the Peyer’s patches and tonsils, the virus replicates and infects cells expressing CD155 such as follicular dendritic cells and B-cells. In non-systemic infections, virus particles are returned to the lumen by an unknown mechanism and the virus returns to the environment through bodily excretions. However, on rare occasions, the poliovirus can enter the bloodstream. Researchers postulate that this occurs as a result of damage to blood vessels (Iwasaki et al, 2002). From the circulation, the poliovirus will eventually end up in the central nervous system via transport across the blood-brain barrier or via retrograde axonal transport. Another newly proposed mechanism of CNS infection is viral transport directly from the Peyer’s patch to the CNS by way of the vagus nerve (Iwasaki et al, 2002). Neuronal cells bear CD155, allowing the poliovirus to infect them and replicate within. As neuronal cells are destroyed, paralytic poliomyelitis can result.
Virus-Receptor Interactions
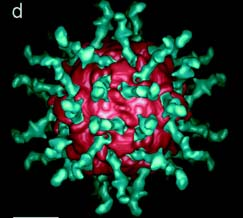
Poliovirus interacts with its receptor on human cells (PVR), also known as CD155, to gain entry into the cell. CD155 is expressed by various cells, including monocytes, macrophages, thymocytes, CNS neurons, M-cells, B-cells, and follicular dendritic cells (Janeway et al., 2001; Iwasaki et al., 2002). CD155 has three extracellular immunoglobulin-like domains, a transmembrane domain, and a cytoplasmic domain. The two types of CD155, resulting from alternative splicing, differ only in the length of their cytoplasmic regions. Immunoglobulin domain 1 contains the sites of viral binding, while the other two extracellular domains play a minor role in affinity (Racanicello 1996). The binding region of domain 1 is known as the C’-C” ridge, and interacts with VP1, VP2, and VP3 (Belnap et al., 2000). Each type of polio strain binds to the receptor somewhat differently, as some mutations will allow one strain to bind but not others.

Fig. 1: Poliovirus (pink) bound to a soluble form of its receptor (green), CD155. Fig. 1d. from Belnap et al., 2000 (3-D structure...). Permission granted.
Certain regions of the virus are important to receptor ligation. A distinctive peak on one axis of the poliovirus is surrounded by a wide, deep chasm deemed ‘the canyon’. This area is where the C’-C” ridge of the receptor inserts and binds. Ligation occurs at multiple locations, as mutations atvarious points in this area reduce affinity.
Following ligation, CD155 induces structural changes in the poliovirus, creating an altered virus commonly called the A particle or 135S state. In this state, the N terminus of VP1, as well as VP4, is expressed on the viral surface as opposed to inside the virus (Belnap et al., 2000). In addition, this form is more sensitive to proteases. These changes give the viral coat a hydrophobic character, allowing it to insert in the host cell membrane. Research strongly suggests that this creates a transmembrane pore in the host cell. After a VP3 plug is removed, RNA can leave the viral capsid and enter the cytoplasm of the host cell (Belnap et al., 2000). Here the virus replicates and causes cell lysis, continuing the spread to other cells.
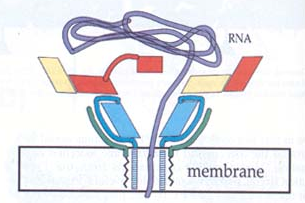
Fig.2: Viral insertion into host cell membrane and suggested mechanism for RNA transfer across cell membrane. Blue=VP1, Yellow=VP2, Red=VP3, and Green=VP4. Note insertion of VP1 into membrane and pore formation resulting in transfer of RNA (Purple). From Belnap et al., 2000 (Molecular Tectonic Model...). Permission requested.
It has also been proposed that when CD155 binds in the viral canyon, it disrupts
a hydrocarbon binding pocket, decreasing sphigosine affinity to the pocket.
Subsequent release of sphigosine destabilizes the capsid, aiding the release
of viral RNA (Racaniello, 1996).
Data has indicated that a physical association between CD44 and CD155 exists and that CD44 may able to transduce an apoptotic signal to the host cell on which it is located, contributing to lysis and cell damage. Additionally, anti-CD44 may be able to block virus binding to CD155 because of the close proximity of the two transmembrane proteins (Gosselin et al., 2003). However, some studies show that CD44's presence has no effect on the binding affinity of poliovirus to CD155, nor does it affect the replication of poliovirus (Racaniello, 1996). As of yet, the role of CD44 in poliovirus is still unknown; future research will reveal whether connections exist.
Types of Polio: Clinical Manifestations
Three main distinctions of polio exist. The mildest is subclinical polio, which sometimes shows no symptoms and accounts for 95% of polio cases. If symptoms are seen, they last a brief period of time and can include fever, malaise, headache, sore throat, or vomiting. Subclinical infections are contained to the gut and lymphoid-associated cells of the gut. Nonparalytic polio shows similar symptoms to subclinical polio, but in addition, patients can experience stiff neck, back and legs, fatigue, muscle tenderness and pain, and diarrhea. These symptoms typically last for 1-2 weeks until the virus is successfully eliminated. Infection can spread beyond gut lymphoid tissues, but has mild effects. Paralytic polio is a severe infection that occurs when a systemic infection moves to the CNS and destroys neuronal cells. Patients experience severe muscle pain, weak muscles, difficulty swallowing and breathing, and abnormal sensations. Paralysis can result quickly, sometimes in a matter of a week. Characterization and severity of paralysis depend on the location of infection within the spinal cord. Typically in paralytic polio, nonparalytic symptoms become progressively more severe, until paralysis results after destruction of 50-60% of the motor neurons in the spine (Todd, 1996; MEDLINEplus, Poliomyeltis, 2003).
Postpoliomyelitis syndrome displays itself 25-30 years after the initial polio infection and is seen in 25% of survivors of paralytic polio. Fatigue, muscle pain, decreased function, and difficulty performing daily activities are characteristic of this syndrome. Manifestations are more severe if initial infection was more severe or occurred later in life. Although postpoliomyelitis could be a result of a reactivation of the poliovirus, it most likely results from increased demand and strain on muscles after initial damage. Polio survirors have imbalanced muscle weaknesses, requiring them to overwork what muscles they are left with. This overuse can result in degeneration of muscle fibers and death of motor nerves, conferring postpoliomyelitis syndrome (Todd, 1996).
Vaccination
Polio cannot be cured once an infection has occurred, but the symptoms can be treated while the virus runs its course of infection. Because polio cannot be cured, vaccination is the only way to avoid the paralytic risks presented by infection. Two polio vaccines exist: inactivated polio vaccine (IPV) and oral polio vaccine (OPV). Both vaccines contain constituents of all three types of poliovirus as well as trace amounts of antibiotics like streptomycin and polymycin (WHO, Adverse…, 2003).
The inactivated polio vaccine was developed by Jonas Salk in 1955 and is most frequently used to vaccinate children in the United States. Vaccination involves four injections of IPV: at 2 months, 4 months, 6-18 months, and 4-6 years (MEDLINEplus, Polio immunization, 2003). In IPV, the polio vaccine has been killed (inactivated) using formaldehyde (WHO, Adverse...,2003). The viral components are taken up and displayed by MHCII on antigen-presenting cells that migrate to lymphoids. B-cells are then stimulated to produce protective levels of antibodies to the poliovirus. After the inactivated form is cleared, memory B-cells remain that allow for a quick response when exposed to poliovirus.
The problem with IPV is that the dead virus is incapable of replication or
synthesis of viral proteins in the cytoplasm of infected cells. This means that
no proteins will be present that can be displayed by MHCI and illicit a CD8
T-cell response. There will be no memory T-cells to fight off exposure to the
virus, only antibodies. Because of this, IPV is less potent than OPV. Also,
because IPV is injected and not orally administered, it confers a much weaker
gastrointestinal immunity than OPV, creating the possibility of polio infection
in the GI tract that could be passed to others.
Despite these concerns, IPV is still an effective vaccination. Possible side effects are almost nonexistent, and include only redness and swelling at the site of injection. IPV cannot cause poliomyelitis, and for this reason, it can be safely given to immunodeficient patients.
The oral polio vaccine, also known as the Sabin vaccine, is a liquid administered by mouth. It contains an attenuated live virus that has been derived from wild-type strains. To make OPV, the virus is grown in a culture of human cells, isolated, and used to infect cells from another organism (typically monkey kidney cells) (WHO, Adverse...,2003). Growth on these monkey cells causes the virus to mutate so as to infect monkey cells most advantageously. This shift renders the virus much weaker at infecting and replicating within human cells, and when taken orally by humans, immunity to polio is induced. Because the virus is still alive, it infects cells and produces viral proteins that can be presented on MHCI, illiciting a CD8 T-cell response. This effectively eliminates the modified virus and creates memory T-cells that easily and quickly kill wild-type poliovirus if exposed (Janeway et al., 2001). OPV’s strength is the chief reason that the United States was able to eradicate polio, and is the vaccine recommended for use in current global eradication efforts.
Fig. 3: Creation of OPV, generalized as creation of any attenuated live virus. With OPV, blue cells are typically monkey kidney cells. Fig. 14.24 from Janeway et al., 2001. Permission requested.
In a study of 9-month olds in Estonia and Finland immunized with OPV and IPV, respectively, the Estonian children who had received OPV had stronger T-cell responses to poliovirus type I. The children receiving OPV also expressed more IFN-gamma when exposed to the poliovirus, which increases the level of response by stimulating increased MHC expression and Ig switching, among other things (Janeway et al., 2001). This study shows conclusively that T-cell immunity to poliovirus is stronger in patients receiving OPV (Juhela et al., 1999).
The problem with OPV is the risk of developing vaccine-associated paralytic poliomyelitis (VAPP). VAPP exhibits the same symptoms as paralytic poliomyelitis, and results in paralysis 4-30 days after vaccination. It is seen in 1/2.4 million people who are administered OPV and has been the cause of every case of polio in the United States since 1979 (MEDLINEplus, Polio immunization, 2003). Strains of OPV mutate quickly in humans, occurring as a result of selective pressure from neutralizing antibodies and temperature constraints (Guillot et al., 2000). If mutation randomly occurs at very precise sites, the live attenuated virus can revert back to the wild-type virus. For example, the type 3 Sabin virus only differs from the wild type virus at 10 nucleotides, illustrating that, although rare, with many quick, precise mutations, reversion is possible. Because of these risks, OPV is never given to immunodeficient patients.
A vaccination schedule that combines IPV and OPV is sometimes used in the United States, presenting an interesting compromise between the two different vaccines. IPV is injected for the first two immunizations, conferring adequate levels of virus-specific antibodies. OPV is administered in the last two doses; at this time VAPP incidence is far less likely because humoral immunity has already been developed. This allows for both humoral and T-cell immunity, reduces risk of VAPP, and involves fewer injections than the complete IPV schedule (Zimmerman et al., 1999).
Eradication
In 1988, a worldwide effort to eradicate polio was initiated by the World Health Organization. This effort, which has employed the use of OPV, has been very successful, reducing the number of cases by 99%. The countries remaining with new cases of polio include India, Nigeria, Egypt, Pakistan, Afghanistan, Niger and Somalia. In February of 2003, India launched an astronomical campaign to immunize 165 million children against polio. Eighty-five percent of new polio cases in 2002 were those reported in India, demonstrating the need for this large-scale campaign (WHO, India.., 2003).
News of Interest
In 2002, Cello et al. reported their assembly of full-length poliovirus cDNA using only oligonucleotides and the known genomic sequence. Experiments with this cDNA showed that it could be transcribed into viral RNA, which could in turn be used to synthesize infectious poliovirus. By showing how (relatively) simple the de novo synthesis of poliovirus is, Cello et al. wanted to show that bioterrorists could easily acquire the poliovirus. This knowledge suggests that although eradication of polio is proceeding successfully, it may not be safe to cease immunization.
Works Cited
Belnap, D., Filman, D., Trus, B. 2000. Molecular Tectonic Model of Virus Structural Transitions: the Putative Cell Entry States of Poliovirus. J. Virology. 74(3): 1342-1354.
Belnap, D. McDermott, B., Filman, D., et al. 2000. Three-Dimensional structure of Poliovirus Receptor Bound to Polivirus. Pub. Nat. Acad. Sci. 97(1):73-78.
Cello, J., Paul, A., Wimmer, E. 2002. Chemical Synthesis of Poliovirus cDNA: Generation of Infectious Virus in the Absence of Natural Template. Science. 297:1016-1018.
Gosselin, A., Simonin, Y., Guivel-Benhassine, F., et al. 2003. Poliovirus-Induced Apoptosis is Reduced in Cells Expressing a Mutant CD155 selected During persistent poliovirus infection in neuroblastoma cells. J. Virology. 77(1): 790-798.
Guillot, S., Carno, V., Cuervo, N. 2000. Natural Genetic Exchanges between Vaccine and Wild Poliovirus Strains in Humans. J. Virology. 74(18):8434-8443.
Hogle, J.M., Chow, M., Filman, D.J. 1985. Three-Dimensional Structure of Poliovirus at 2.9 angstrom resolution. Science. 229: 1358-1365.
Iwasaki, A., Reinhold, W., Mueller, S., et al. 2002. Immunofluorescence Analysis of Poliovirus Receptor Expression in Peyer’s Patches of Humans, Primates, and CD155 Transgenic Mice: Implications for Poliovirus Infection. J. Infectious Diseases. 186: 585-592.
Janeway, C., Travers, P. Walport, M., Shlomchik, M. 2001. Immunobiology: The Immune System in Health and Disease. New York, New York: Garland Publishing.
Juhela, S., Hyoty, H., Uibo, R. 1999. Comparison of Enterovirus-specific Cellular Immunity in Two Populations of Young Children Vaccinated with Inactivated or Live poliovirus vaccines. Clinical and Experimental Immunology. 117(1): 100-105.
Racaniello, V. 1996. Early Events in Poliovirus Infection: Virus-receptor Interactions.
Proc. Natl. Acad. Sci. 93: 11378-11381.
Todd, J. 1996. Post-Polio Syndrome: A Literature Review & Case Report. Gerontology Manual. Tacoma, WA. School of Occupational Therapy and Physical Therapy, University of Puget Sound. http://otpt.ups.edu/Gerontological_Resources/Gerontology_Manual/Todd_J.html Accessed 2003 24 Apr.
U.S. National Library of Medecine & National Institutes of Health. Last updated 2002 27 Feb. MEDLINEplus Medical Encyclopedia: Poliomyelitis. http://nlm.nih.gov/medlineplus/ency/article/001402.htm Accessed 2003 17 Apr.
U.S. National Library of Medecine & National Institutes of Health. Last updated 2001 16 Mar. MEDLINEplus Medical Encyclopedia: Polio immunization. <http://www.nlm.nih.gov/medlineplus/ency/article/002030.htm> Accessed 2003 17 Apr.
World Health Organization. Adverse Events Following Poliomyelitis Vaccine.
http://www.who.int/vaccines-diseases/safety/infobank/polio.shtml Accessed 2003
24 Apr.
World Health Organization. 2003. India launches largest ever campaign to tackle polio epidemic. http://www.who.int/mediacentre/releases/2003/pr8/en/ Accessed 2003 16 Apr.
Zimmerman, R., Spann, S. 1999. Poliovirus Vaccine Options. American Academy of Family Pysicians. http://www.aafp.org/afp/990101ap/113.html Accessed 2003 16 Apr.
Questions, Comments, or Suggestions?: E-mail Sara
Return to Immunology Home Page