This web page was produced as an assignment for an undergraduate course at Davidson College.
Fas
Overview
Fas is a cell-surface receptor protein that when stimulated, induces apoptosis in the Fas-bearing cell. Apoptosis triggered by Fas ligation is crucial within the immune system, as the ligand for Fas, FasL, is chiefly expressed on cytotoxic T-cells and TH1 cells. Fas cell death is involved in an array of immune-system functions, including elimination of used effector cells and elimination of peripheral autoreactive lymphocytes. Mutations in Fas and/or its ligand cause autoimmune disease.
General Characteristics of Fas
Fas is a cell-surface receptor protein with an extracellular region, one transmembrane domain, and an intracellular region. Other names for Fas include CD95 and APO-1. Fas belongs to the tumor necrosis factor/nerve growth factor (TNF/NGF) cytokine receptor family, whose members are characterized by conserved cysteine-rich extracellular domains, of which Fas has three. The intracellular domains of TNF/NGF receptor family members do not show the homology of the extracellular regions, save for similarities between TNF-R1 (whose ligand is TNF-alpha) and Fas. (Nagata et al., 1995). Other members of the TNF/NGF family include CD40, NGF-R and TNF-R2. Human Fas has been localized to chromosome 10 and consists of 9 exons encoding 325 amino acids (Nagata et al., 1995).
Figure 1: Interactive CHIME of Fas. From RCSB Protein Data Bank. http://www.rcsb.org/pdb/cgi/explore.cgi?job=graphics&pdbId=1DDF&page=0&pid=84401047963207
Expression of Fas
Fas activity is governed by interaction with its ligand (FasL),
which is expressed on the surface of activated cytotoxic T-cells as well as
some activated TH1 cells. Cytotoxic T-cells and TH1
cells secrete IFN-gamma, which has been shown to induce higher levels of Fas
expression on lymphocytes (Pouly et al., 2000). These could be infected
lymphocytes or non-infected activated lymphocytes, however, Fas expression is
most significant in activated lymphocytes (Janeway et al., 2001). Fas
is widely expressed in tissues and among different cell types, but studies have
shown the highest Fas levels in the thymus, liver, heart, lung, kidney, and
ovary (Nagata et al., 1995; Itoh et al., 1991). In short,
Fas is expressed weakly on a variety of both lymphoid and non-lymphoid cells,
but cells in the immune system are most sensitive to the effects of Fas and
to up-regulation of Fas by IFN-gamma and lymphocyte activation.
Fas as a death receptor
Activation of Fas through binding to its ligand or Fas antibody induces apoptosis, which has been confirmed by many experiments. Itoh et al. (1991) saw the DNA fragmentation and surface blebs assosciated with apoptosis after incubating a particular cell line expressing Fas with anti-Fas antibody. The parent cells, which did not express Fas, showed neither DNA fragmentation or surface blebs when treated with the same anti-Fas antibody. Ligation with FasL, anti-Fas antibody, or secreted FasL causes transduction of death signals through Fas. When FasL is secreted, it can bind to Fas on the surface of the same T-cell (autocrine signaling), or it can bind to Fas on the surface of another nearby cell (paracrine signaling) (Krammer et al.,1994).
Stimulation of Fas can also induce activation of an acidic sphingomyelinase (SMase), which leads to production of ceramide, a regulator of cell stress (O'Connell, 2001). Higher stress levels play an additional role in apoptosis.
How is the death signal transduced?
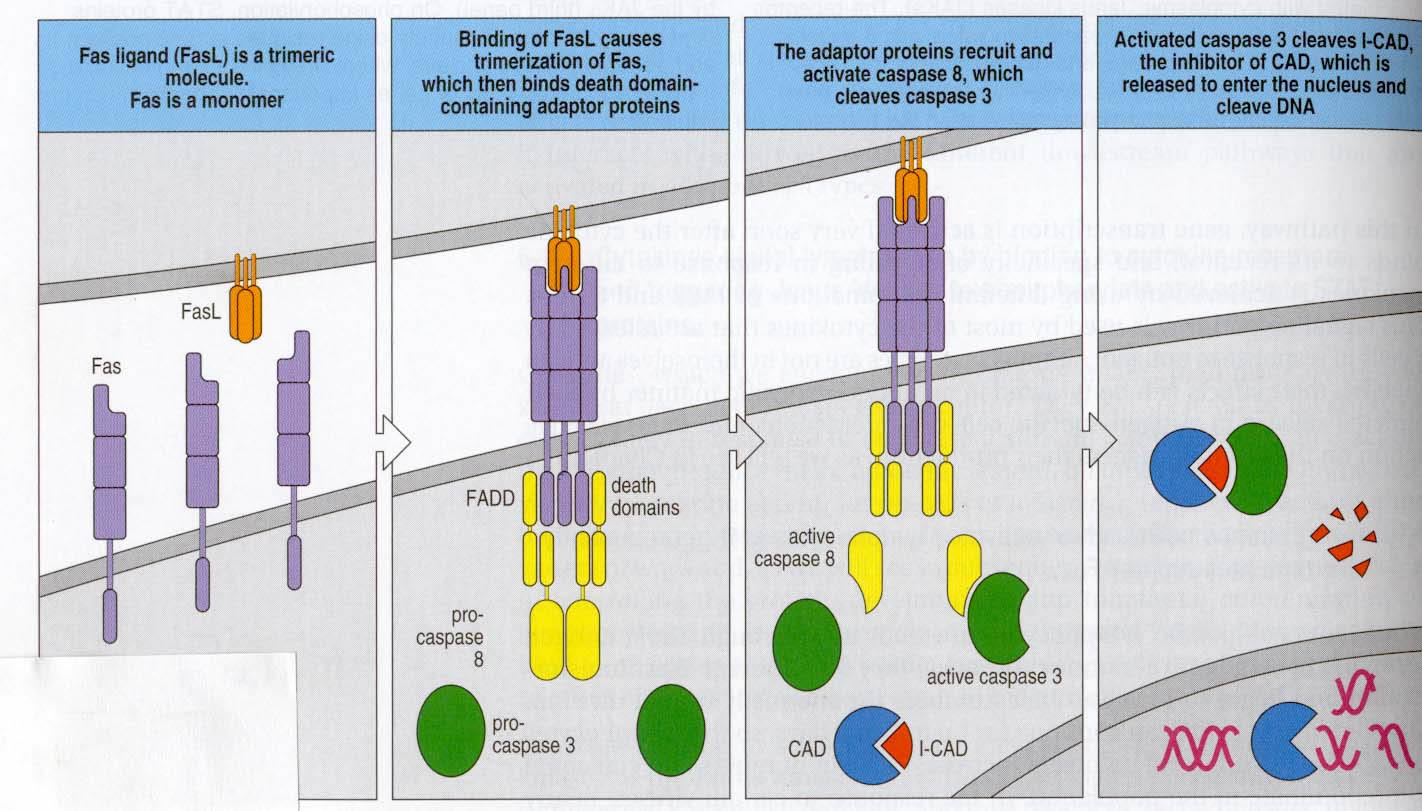
In order for signal transduction to occur, cross-linking of Fas with its ligand must occur. Fas trimerizes to properly bind to its ligand, which exists as a trimer. This creates a clustering of Fas that is necessary for signaling. In its intracellular region, Fas contains a conserved sequence deemed a “death domain”. An adaptor protein, FADD, interacts with the death domain on the Fas receptor. Subsequent binding to another region of FADD by pro-caspase 8 promotes grouping of pro-caspase 8 molecules bound to each of the clustered FADD proteins. This entire cluster is sometimes called a death-inducing signaling complex, or DISC (Hueber et al., 2000). Pro-caspase 8 transactivates itself once grouped, cleaving and releasing active caspase 8 molecules intracellularly. These in turn cleave pro-caspase 3, which in this activated form, is capable of denaturing I-CAD, thereby activating CAD, a DNase that enters the nucleus and fragments DNA (Janeway et al., 2001). These DNA fragments would all be close in size and would appear similar to those seen in the Itoh et al. (1991) experiment described above that confirmed the induction of apoptosis by Fas.

Figure 2: The Fas signaling pathway, displayed exactly as described above. Cell bearing FasL is a cytotoxic T-cell or a TH1 cell. Note that FasL exists as a trimer and Fas must trimerize in order to bind. (Fig. 6.23 from Janeway et al., 2001. Permission requested.)
Fas and TNF-R1 both signal apoptosis, and the homology between Fas and TNF-R1
described earlier would seem to suggest that the two signal apoptosis in the
same fashion when TNF-R1 binds to TNF-alpha. However, there is some controversy
surrounding the inferred similarities between the two processes. One view is
that the pathways are similar, but that the Fas pathway is the more important
of the two (Janeway et al., 2001). However, others maintain that the
two pathways are fundamentally different. Nagata et al. (1995) showed
that certain substances block TNF-R1 death signaling that have no effect on
Fas death signaling, which also occurs faster than TNF-R1 death signaling. Nagata
argued that these differences indicate separate pathways for each signaling
mechanism. In addition, it was shown that each pathway exhibited differential
activation of NFkB, a nuclear transcription factor (Lu et al., 2002).
Cytotoxic T-cells also use a perforin-granzyme-based pathway to induce apoptosis on their targets. This was discovered before the Fas pathway, but it was apparent that an alternate path existed. For one, the perforin-granzyme pathway is Ca++ dependent, but in the absence of Ca++, some CD8 cytotoxicity was still seen (Janeway et al., 2001). Also, TH1 cells have no granules (a key component of the perforin-granzyme pathway), but are still sometimes capable of inducing apoptosis (Janeway et al., 2001). Thes two observations showed that another pathway existed, which turned out to be the Fa pathway.
In what significant situations does Fas signaling occur?
Fas is predominantly responsible for eliminating activated lymphocytes after they have performed their function in the adaptive immune response. When lymphocytes are activated, they proliferate and differentiate rapidly, creating a staggering number of armed effector cells. The adaptive immune response to some viruses stimulates producton of so many virus-specific cytotoxic T-cells that they account for 50% of CD8 T-cells at one point during the response (Janeway et al., 2001). As a reminder, in T-cells, this is growth is largely stimulated by IL-2 as well as CD28, B7 ligation (Janeway et al., 2001). It is obvious that, in order to maintain homeostasis, this large number of armed effector cells must be eliminated, leaving only a small number behind to confer immunological memory. For T-cells, once they are activated by antigen, Fas expression on their cell surface is upregulated. However, these T-cells only become sensitive to the Fas pathway after a few days of activation, once anti-apoptotic Bcl-2 levels decrease and the T-cells are more sensitized to apoptosis (O'Connell, 2001). An identical pathway is present in B-cells, which is important, because accumulation of B-cells can result in autoantibody production (Nagata et al., 1995).
Additionally, Fas downregulates the immune response by blocking the release of Ca++, thereby inhibiting release of substances causing immune upregulation, like IL-2 (O'Connell, 2001).
TH1 cells can activate macrophages to kill engulfed bacteria, but sometimes these macrophages are incapable of activation and remain infected. If such a macrophage expresses Fas, FasL on the surface of the TH1 cell can bind, signaling the macrophage to undergo apoptosis. This destruction causes release of the engulfed bacteria, allowing it to be picked up by a new and hopefully more effective macrophage.
It has also been seen that Fas plays a role in eliminating self-reactive B and T cells in the periphery. It is possible for some autoreactive B and T cells to survive negative selection if their self ligand is not expressed in the thymus. The Fas pathway helps to eliminate some of these escapees. For example, autoreactive B-cells binding to self in the periphery will receive no second signal and will enter a state of anergy. If during anergy, they are presented with the rare opportunity to bind to a TH1 cell specific to self antigen, signal transduction and apoptosis through the Fas pathway could occur (Janeway et al., 2001). This would eliminate the self-reactive B-cell, as well as any possibility of developing autoantibodies, making it clear why Fas-deficient mice and humans sometimes express autoantibodies.
What happens when Fas is mutated?
When Fas is mutated in humans, a disorder termed autoimmune lymphoproliferative syndrome (ALPS) results. This syndrome is characterized by massive swelling of lymph nodes (lymphoadenopathy), autoimmune problems, and abnormally high numbers of double negative T-lymphocytes. An investigation of five children with this disorder revealed that each had a different mutation in the gene encoding Fas (Fisher et al., 1995). Other less common symptoms include production of autoantibodies and nephritis, which vary depending on the specific mutation as well as the cell background of the patient. Some of the mutations that cause this disorder are recessive, however, others are inherited in a dominant fashion. All five of the patients that Fisher et al. (1995) reported had inherited ALPS in a dominant manner. Dominant inheritance is most likely conferred because of the need for Fas to trimerize; if just one of the substituents is mutated, the trimer will still be incapable of forming. Other researchers, however, have found cases of ALPS inherited recessively (van der Burg et al., 2000). Mutations in Fas are most commonly seen in exons 7 and 9, which encode the intracellular portion of Fas containing the all-important death domain. These mutations also showed a more penetrant phenotype than mutations elsewhere in Fas, indicating that their effect is more compromising (Jackson et al., 1999).
Why do these mutations cause the ALPS phenotype? These patients certainly have problems in the normal apoptotic pathway induced by Fas. In one study, nine independent heterozygous mutations in different individuals altered the death domain, rendering it impossible for FADD to bind to Fas intracellularly and initiate the necessary caspase cascade (Martin et al., 1999). It should be noted that 17% of patients with ALPS do not have a mutation in Fas, so the syndrome can be caused via other defective genes, most likely genes governing apoptosis in some way (Straus et al., 1999). One possibility would be a defect in BCL-2, which inhibits apoptosis when expressed.
Lpr and gld mice have spontaneous mutations in Fas and FasL, respectively, and both exhibit phenotypic similarities to ALPS patients. One problem in lps and gld mice is impaired colnal deletion in the periphery, which results in an autoimmune syndrome. In addition, these mice show impaired elimination of T-cells whose function has been exhausted, with abnormally high T-cell counts in the spleen and lymph noes (O'Connell, 2001). These mice strains were not only instrumental in determining the function and interaction of Fas and FasL, but serve as useful test subjects when studying theraputic applications of Fas.
Selected Theraputic Applications of Fas
HIV/AIDS - In AIDS, healthy CD4+ T-cells are depleted. Limited results suggest that Fas-mediated apoptosis may be responsible for this destruction (Krammer et al., 1994). In support of this hypothesis, it was shown that Tat, a protein made by HIV, upregulates expression of FasL, making CD4 T-cells more susceptible to apoptosis (Ensoli et al., 1995). Some drugs, such as L-carnitine, have been shown to increase CD4 T-cell counts and decrease levels of ceramide, an apoptosis mediator that results from the stimulation of Fas. To induce these effects, L-carnitine binds to Fas and renders it incapable of signaling. (Mutomba et al., 2000).
Diabetes - One characteristic of diabetes progression is the destruction of beta cells. When researchers engineered mice with beta cells expressing mutated Fas, "spontaneous diabetes was significantly delayed" (Savinov et al., 2003). This suggests the possibility to use altered Fas to slow or prohibit the progression of diabetes.
Hepatitis - When normal mice were injected with an agonistic Fas antibody, acute liver failure (fulminant hepatitis) and death followed after three days. Another strain of mice with mutated Fas in their hepatocytes (created by an siRNA injection) were injected with the same agonistic Fas antibody, and 81% survived for the ten day observation period (Song et al., 2003).
Leukemia - Fas can play a role in eliminating cancerous cells. In mice with T-cell leukemia, apoptosis of the T-cell leukemia cells was induced by anti-Fas. These mice survivied longer than mice not exposed to anti-Fas, but not all T-cell leukemia cells were eliminated. Researchers saw similar results for B-Cell tumors as well. Because not all cancerous cells were eliminated when exposed to anti-Fas, some tumor cells resist Fas-associated death. A suggested resistance mechanism is that resistant cells switch to producing soluble Fas, thereby escaping cell-surface apoptotic signaling (Krammer et al., 1994).
Toxic Epidermal Necrolysis (TEN) - When patients with this skin disease were exposed to a naturally occuring anti-Fas immunoglobulin, their keratinocytes were not able to undergo apoptosis. This subsequently halted progression of the skin disease (Viard et al., 1998).
Interesting Links
NIH Introduction for ALPS Patients and Families
References
Ensoli, B., Barillari, G., Salahuddin, S., et al. 1995. Tat protein of HIV-1 Stimulates Growth of cells Derived from Kaposi's Sarcoma Lesions of AIDS Patients. Nature. 345:84-86.
Fisher, G.H., Rosenberg, F.J., Straus, S.E., et al. 1995. Dominant Interfering Fas Gene Mutations Impair Apoptosis in a Human Autoimmune Lymphoproliferative Syndrome. Cell. 81:935-946.
Hueber, A.-O, Zornig, M., Lyon, D., Suda, T., Nagata, S., Evan, G. I. 1997. Requirement for the CD95 Receptor-Ligand Pathway in c-Myc-Induced Apoptosis. Science. 278: 1305-1309.
Itoh, N., Yonehara, S. Ishii, A., et al. 1991. The Polypeptide Encoded by the cDNA for Human Cell Surface Antigen Fas Can Mediate Apoptosis. Cell. 66: 233-243.
Jackson, C., Fischer, R. Hsu, A., et al. 1999. Autoimmune Lymphoproliferative Syndrome with Defective Fas: Genotype Influences Penetrance. Am. J. Human Genetics. <http://www.journals.uchicago.edu/AJHG/journal/issues/v64n4/980898/980898.html> Accessed 2003 20 Mar.
Janeway, C., Travers, P. Walport, M., Shlomchik, M. 2001. Immunobiology: The Immune System in Health and Disease. New York, New York: Garland Publishing. p. 214-215, 313-314.
Krammer, P. Dhein, J., Walczack, H., et al. 1994. The Role of APO-1-Mediated Apoptosis in the Immune System. Immunological Reviews. 124:175-191.
Lu, B., Wang, L., Medan, D. Toledo, D., et al. 2002. Regulation of Fas (CD95)-Induced Apoptosis by Nuclear Factor-kB and Tumor Necrosis Factor-a in Macrophages. Am J Physiol Cell Physiol. 283(3): C831-C838. <http://ajpcell.physiology.org/cgi/content/full/283/3/C831> Accessed 2003 17 Mar.
Martin, D., Zheng, L., Siegel., R., et al. 1999. Defective CD95/APO-1/Fas Signal Complex Formation in the Human Autoimmune Lymphoproliferative Syndrome, Type Ia. Proc. Nat. Acad. Sci. 96:4552-4557. <http://www.pubmedcentral.gov/articlerender.fcgi?tool=pubmed&pubmedid=10200300> Accessed 2003 20 Mar.
Mutomba, M., Yuan, H., Konyavki, M., et al., 2000. Regulation of the Activity of Caspases by L-carnitine and palmitoylcarnitine. FEBS Latters. 478(1-2):119-125. <http://www.ncbi.nlm.nih.gov/entrez/utils/fref.fcgi?http://linkinghub.elsevier.com/retrieve/pii/S0014579300018172> Accessed 2003 21 Mar.
Nagata, S., Goldstein, P. 1995. The Fas Death Factor. Science. 267: 1449-1456.
O'Connel, J. 2001. Role of Fas-FasL in Imflammatory Diseases. Expert Reviews in Molecular Medecine. <http://www-ermm.cbcu.cam.ac.uk/01003969h.htm> Accessed 2003 20 Mar.
Pouly, S., Becher, B., Blain, M., Antel, J.P. 2000. Interferon-gamma Modulates Human Oligodendrocyte Susceptibility to Fas-mediated Apoptosis. J Neuropathol Exp Neurol. 59(4):280-286.
Savinov, A., Tcherepanov, A., Green, E., et al. 2003. Contribution of Fas to Diabetes Development. Proc Nat Acad Sci. 100:628-632. <http://www.pnas.org/cgi/content/full/100/2/628> Accessed 2003 20 Mar.
Song, E., Lee, S., Wang, J., et al. 2003. RNA Interference Targeting Fas Protects Mice from Fulminant Hepatitis. Nature Med. 9:347-351.
Straus, S., Sneller, M., Dale, J. 1999. Autoimmune Lymphoproliferative Syndrome (ALPS): An Introduction for ALPS patients and their Families. <http://www.niaid.nih.gov/publications/alps/alps.htm#a>Accessed 2003 20 Mar.
van der Burg, M., de Groot, R., Comans-Bitter, W., et al. 2000. AutoimmuneLymphoproliferative Syndrome (ALPS) in a Child From Cansanguineous Parents: A Dominant or Recessive Disease? Pediatric Res. 47: 336-343. <http://www.pedresearch.org/cgi/content/full/47/3/336> Accessed 2003 19 Mar.
Viard, I., Wehrli, P., Bullani, R., et al.1998. Inhibition of Toxic Epidermal Necrolysis by Blockade of CD95 with Human Intravenous Immunoglobulin. Science. 282:490-493.
Questions, Comments, or Suggestions?: E-mail Sara