This page is part of an undergraduate course at Davidson College.
Human Immune Protein FcRn: IgG Transcytosis and Catabolism
FcRn in the Immune System:
FcRn is one of the answers to the key question "how does a fetus gain the immune response it needs, until its own immune system develops after birth"? One answer for humans is IgA secreted through the mother’s colostrum; IgA is then transcytosed across the epithelia of the gut and enters the bloodstream. In addition to oral reception, antibodies are also delivered in other ways to the human fetus: for example, IgG is delivered directly into the fetal bloodstream across the placenta. Transcytosis of antibodies from mother to fetus is conferred by maternal FcRn, which stands for Fc receptor neonatal, and actively transports IgG across the placenta from mother to child.
Additionally, FcRn controls the catabolism of IgG in the adult body, allowing IgG to be recycled to the cell surface and back into the bloodstream. This recycling and regulation of catabolism gives IgG a much longer half-life than other antibodies (Janeway et al., 2001).
Structure
Structurally, FcRn is made of two polypeptide chains: one is
B-2 microglobulin and the other is very similar to the Mhc I alpha-chain, including
the binding cleft. Unlike Mhc I, though, the binding cleft is occluded and the
antibodies are bound to a separate area of the FcRn molecule by the antibody
Fc region. Specifically, FcRn requires the amino acids Ile253, His310, and His435
in that order in IgG for proper binding (Simister and Story 1997). These aminos
are conserved in human IgG, and it has been shown in mutant IgG’s that
either lack these aminos or have a different order of aminos, the half-life
of the antibody is much shorter than wild-type IgG (Junghans and Anderson, 1996).
FcRn binds IgG at the Fc region of the antibody, and two FcRn’s bind to
one IgG for transcytosis across the placenta. FcRn binds IgG between the CH2
and CH3 regions using all three of the FcRn alpha domains. Early research suggested
that 2 FcRn molecules bind 1 IgG for transcytosis, and this was confirmed by
X ray crystallography (Ghetie and Ward, 2002).
Image 1: Chime image of IgG transport protein FcRn.
Transcytosis
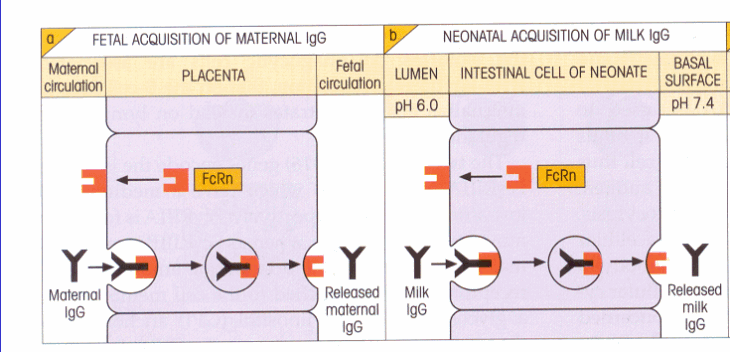
Transcytosis by FcRn occurs in multiple places in humans: carrying IgG into the fetal bloodstream, in the adult liver, and from blood to milk in the lactating mammary glands, for some. Evidence for FcRn as the mediator of transcytosis has been found by northern blotting and finding expression of FcRn on the placental trophoblast as well as the endothelia of the liver and intestines in adults (Ghetie and Ward, 2002). The mechanism underlying transcytosis of IgG across the placenta is regulated by pH. FcRn binds IgG at pH 6.0, which is the pH in the maternal bloodstream, and releases at pH 7.4, which is the pH in the fetal bloodstream. Trophoblast cells in the placenta uptake fluid-phase IgG by pinocytosis, maternal apical vesicles are released which are acidic and cause binding between FcRn and IgG, FcRn transports the antibodies across the cells, and upon reaching the fetal bloodstream where pH is 7.4 the IgG is released. FcRn then moves back across the trophoblast cells to the mother, to transcytose more IgG to the fetus (Simister and Story, 1997). This is illustrated in Figure 1, below.
Figure 1: Box "a" shows transport of IgG across the placenta in humans; box "b" shows transport across the intestine in rodents. Courtesy University of Glasgow, Division of Immunology, Infection, and Inflammation.
Catabolism
It is known that IgG antibodies have a very long half-life
in the human body, and it is believed that FcRn mediates this longevity by preventing
degradation. It is proposed that IgG taken up into endothelial cells will either
be bound by FcRn and released back into the circulation, or if they are not
bound they will be degraded. It is believed this ability of FcRn to bind and
release functional IgG’s back into the bloodstream acts like recycling,
allowing IgG antibodies to outlive other antibodies.
Fundamentally, IgG catabolism is transcytosis either, back into the bloodstream,
or if not to the bloodstream then to degradation. Two theories exist for the
specificity of IgG catabolism: the selective hypothesis and the random hypothesis.
The selective hypothesis states that IgG’s are degraded following a structural
or conformational change. The random hypothesis, which is the currently believed
model, states that selection of IgG for degradation is random-some IgG will
be degraded and some will be recycled independent of their age (Ghetie and Ward,
2002).
Figure 2: Box "a" diagrams the catabolism of IgG by FcRn in endothelial cells; box "b" shows transcytosis across endothelial cells. Courtesy University of Glasgow, Division of Immunology, Infection, and Inflammation.
What is the gene for FcRn?
In rodents the FcRn heavy chain gene, designated Fcgrt, maps to chromosome 7, which is outside the Mhc region (Ahouse et al. 1993). In humans, it has been shown by fluorescence in situ hybridization that the human gene, FCGRT, maps to 19q13.3, which is also outside the Mhc region (Kandil et al. 1996). So, despite FcRn's strong resemblance to Mhc in mice and men, both Fcgrt and FCGRT, which bear strong homology, are encoded outside the HLA-domain.
Rodents and humans have been the most extensively studied models for FcRn, and in these models the gene for the FcRn heavy chain is symbolized Fcgrt. It appears that homology between the genes in both animals is very strong. In one experiment, researchers cloned a cDNA encoding the human gene and named it FcRn (Story et al. 1994) . In a follow-up experiment, different researchers isolated human genomic clones for the human FcRn gene using 2 murine Fcgrt probes (Kandil et al. 1996). The sequences of the genomic clones are identical to the cDNA sequence described by Story et al. (1994).
What are the effects of not expressing functional FcRn?
In individuals that do not have functional FcRn, two phenotypes would be present: first would be an inability to transfer FcRn to a fetus, and the second would be an over-catabolism of IgG, leading to a much shorter lifespan for the antibody.
Junghans et al. (2001) postulated that "long-range cis inactivation" of the FCGRT gene is responsible for over-catabolism of IgG in some disorders, like myotonic dystrophy. These disorders, and particularly myotonic dystrophy, are notable for the short half-life of IgG in individuals afflicted with them.
In knockout mice for FcRn, the catabolism rate for IgG may be accelerated up to 10 times compared to wildtype animals in which FcRn is expressed normally (Junghans and Anderson, 1996). This protective role of FcRn is directly responsible for making IgG the the immune protein with the longest lifespan.
References
Ahouse, J. J.; Hagerman, C. L.; Mittal, P.; Gilbert, D. J.; Copeland, N. G.; Jenkins, N. A.; Simister, N. E. 1993. Mouse MHC class I-like Fc receptor encoded outside the MHC. J. Immun. 151: 6076-6088.
Ghetie, V., and Ward, E.S. 2002. "Transcytosis and Catabolism of Antibody." Immunologic Research 25(2): 97-113.
Janeway, C. A. Jr., Travers, P., Walport, M., and Shlomchik, M. J. Immunobiology 5. New York: Garland Publishing, 2001.
Junghans, R. P.; Ebralidze, A.; Tiwari, B. 2001. Does (CUG)n repeat in DMPK mRNA 'paint' chromosome 19 to suppress distant genes to create the diverse phenotype of myotonic dystrophy? A new hypothesis of long-range cis autosomal inactivation. Neurogenetics 3: 59-67.
Junghans, R. P.; Anderson, C. L. (1996). The protection receptor for IgG catabolism is the beta-2-microglobulin-containing neonatal intestinal transport receptor. Proceedings of the National Academy of Sciences 93: 5512-5516.
Kandil, E.; Egashira, M.; Miyosi, O.; Niikawa, N.; Ishibashi, T.; Kasahara, M. 1996. The human gene encoding the heavy chain of the major histocompatibility complex class I-like Fc receptor (FCGRT) maps to 19q13.3. Cytogenet. Cell Genet. 73: 97-98.
Simister, N.E., and Story, C.M. 1997. "Human placental Fc receptors and the transmission of antibodies from mother to fetus." Journal of Reproductive Immunology 37: 1-23.
Story, C. M.; Mikulska, J. E.; Simister, N. E. 1994. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J. Exp. Med. 180: 2377-2381.
Davidson College Biology Bio 307
Spring Semester 2003
Contact Peter Leese or View Immunology at Davidson College