This web page was produced as an assignment for an undergraduate
course at Davidson College.
Fas
Click here to see an enlarged
chime of the Fas death domain and the figure legend.
Fas is my favorite immunology protein because it
initiates one of the most important pathways to programmed cell death, or apoptosis,
in the body. Apoptosis is important in cell-mediated immune response, autoimmune
tolerance, and cancer control. Fas is a surface receptor that is expressed throughout
the body. The Fas ligand is expressed mostly on activated T cells, natural killer
cells, and microglia, but is sometimes found on other cells of the immune system.
When Fas binds to its ligand, a caspase cascade is initiated within the cell
that eventually leads to its death (Maher et al. 2002). The first half
of my website will give more details about the structure and function of the
Fas protein in the immune system. The second half will explore the clinical
aspects of Fas.
Structure

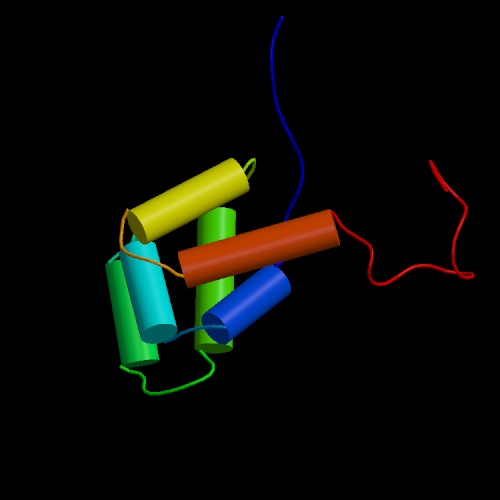 Fas
(also referred to as CD95 or APO-1) is a 48-kDa (319 amino acids) member of
the TNF/nerve growth factor receptor family. It has three regions: a N-terminal
extracellular domain, a single transmembrane domain, and a cytoplasmic domain.
The extracellular domain has three repeats of a cysteine-rich subdomain common
to all members of the TNF receptor family. The extracellular domain also
contains two glycosylation sites where sugars probably bind. The transmembrane
domain is 17 amino acids long (Itoh et al. 1991). Within the cytoplasm
lies an abundantly charged region known as the death domain. Figures 1
and 2 (click to see enlarged images
and figure legend) show models of the Fas death domain. The structure
consists of six antiparallel a helices capable
of self-association. This capability is extremely important to internal
cell signaling following Fas binding, as you will see below.
Fas
(also referred to as CD95 or APO-1) is a 48-kDa (319 amino acids) member of
the TNF/nerve growth factor receptor family. It has three regions: a N-terminal
extracellular domain, a single transmembrane domain, and a cytoplasmic domain.
The extracellular domain has three repeats of a cysteine-rich subdomain common
to all members of the TNF receptor family. The extracellular domain also
contains two glycosylation sites where sugars probably bind. The transmembrane
domain is 17 amino acids long (Itoh et al. 1991). Within the cytoplasm
lies an abundantly charged region known as the death domain. Figures 1
and 2 (click to see enlarged images
and figure legend) show models of the Fas death domain. The structure
consists of six antiparallel a helices capable
of self-association. This capability is extremely important to internal
cell signaling following Fas binding, as you will see below.
Transduction Pathway
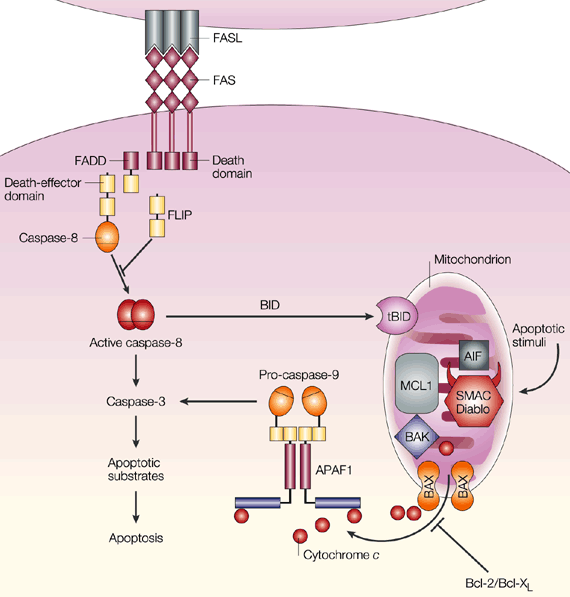 When
a cell expressing Fas encounters another cell expressing Fas ligand, the
receptor molecules trimerize in order to bind the ligand. Trimerization of
the death domains in the cytoplasmic regions of each molecule is the signal for
activation. After activation, the death domains are capable of
interacting with each other, which leads to the recruitment of FADD, or
Fas-associated death domain protein. In addition to the death domain, FADD
has a death-effector domain (DED) capable of recruiting and splicing
procaspase-8 into its active form, caspase-8 (Krammer 2000). The
concentration of caspase-8 determines which of two paths are taken towards
apoptosis.
When
a cell expressing Fas encounters another cell expressing Fas ligand, the
receptor molecules trimerize in order to bind the ligand. Trimerization of
the death domains in the cytoplasmic regions of each molecule is the signal for
activation. After activation, the death domains are capable of
interacting with each other, which leads to the recruitment of FADD, or
Fas-associated death domain protein. In addition to the death domain, FADD
has a death-effector domain (DED) capable of recruiting and splicing
procaspase-8 into its active form, caspase-8 (Krammer 2000). The
concentration of caspase-8 determines which of two paths are taken towards
apoptosis.
If caspase-8 levels are high, it directly cleaves caspase-3
(Maher et al. 2002). Caspase-3 inactivates the inhibitor of CAD (caspase-activatable
DNAse). CAD is then free to enter the nucleus and cleave DNA into lengths
of approximately 180 base pairs (Ju,
Matsui, and Ozdemirli 1999).
If caspase-8 concentration is low, it
truncates Bid into tBID, a form capable of releasing cytochrome c from
the mitochondria. Cytochrome c interacts with proteins such as
Apaf-1, dATP, and procaspase-9 to produce active caspase-9. Caspase-9 then
cleaves caspase-3, which activates CAD in the same way as it does in the first
pathway. Apoptosis inhibitors Bcl-2 and Bcl-XL block apoptosis
through the BID-mediated pathway but not the direct (caspase cascade) pathway
(Maher et al. 2002).
Another group of inhibitors, known as FLIPs (FLICE-inhibitory
proteins-- caspase-8 used to be called FLICE), contain two DED domains capable
of binding to the Fas--FADD complex. In this way they prevent the
recruitment of caspase-8 and inhibit both pathways to apoptosis. FLIPs
were first identified in a class of herpes virus, but two human homologues have
recently been identified (Krammer 2000).
Figure 3 (at left)
summarizes the two Fas-mediated apoptotic pathways. Click on the image to
see a larger version and the figure legend.
Role in Immune Response
Cytotoxic T cells and natural killer cells are responsible for
destroying cells infected with intracellular cytoplasmic pathogens and cancer
cells. It is important that these cells die by apoptosis so that the pathogen
does not survive. Necrosis, which occurs when a cell dies due to infection
or other types of damage, allows the pathogen to safely exit the cell and infect
others. During apoptosis, CAD cleaves DNA and toxic proteases are released into
the cytoplasm. The toxic environment sacrifices the cell but usually kills
the pathogen with it. This pathway is initiated through Fas in conjunction
with the perforin/granzyme pathway.
Both methods of inducing apoptosis are activated when the T cell
receptor binds its specific antigen, at which point the cell proliferates and
FasL, perforin, and granzyme are expressed. When the active T cell encounters
its antigen again, FasL stimulates the target cell to began a caspase cleavage
cascade as described above (Nagata and Golstein 1995). Perforin attacks
the cell by creating a pore in the nuclear membrane. Granzymes then diffuse
through the pore into the cell cytoplasm, where they are capable of activating
caspases in a similar way to the Fas pathway (Darmon, Nicholson, and Bleackley
1995). Whether there is a preference for one method or another depending
on the infected cell type or pathogen has not been determined. A similar
mechanism occurs during Fas-induced apoptosis by natural killer cells, although
the details are less clear ( Nagata 1997).
Role in Immunoregulation
The immune system must be tightly controlled in order to prevent
excessive tissue damage and cell accumulation. Developing T cells in the
thymus must be destroyed if they do not produce a functional T cell receptor
or are unable to bind with the right affinity for self MHC class I molecules.
After infection, lymphocyte levels must return to normal, meaning that many
of the newly formed effector cells will die. Some areas of the body, such
as the ovaries, testis, and retina, cannot survive the damage they would sustain
as a result of an inflammatory immune reaction. These areas are not subject
to immune responses, and for that reason are known as immune privileged.
The Fas pathway has been implicated in all of these areas of immune
regulation. A Fas knockout mouse strain, known as lpr (lymphoproliferation)
has been developed that shows significant defects in these areas. Another
strain expresses the receptor, but a point mutation produces a misfolded form
incapable of transducing signals. The mutant strain is referred to as
lprcg. Both the knockout and the mutation produce similar effects.
These include: excessive numbers of CD4-/CD8- T cells, enlarged spleen and lymph
nodes, and low red blood cell and platelets due to autoimmune reactions (Kimura
and Matsuzawa 1994).
Similar effects are observed in patients with autoimmune lymphoproliferative
syndrome (ALPS). Most patients are heterozygous for a mutant allele that
disrupts the a3-helix of Fas. The mutation
results in decreased recruitment of FADD and caspase-8. It is not fully
understood why the mutant allele would inhibit the functional allele (loss of
function is >50%), but it has been suggested that the Fas signaling complex
may require that some components be preassembled, allowing the unfolded protein
to interfere with FADD binding (Martin et al. 1999).
CD4-CD8- T cells are immature cells that have not produced a
functional b-chain receptor. Normally they
die when they don't receive a survival signal during positive selection.
Their presence in the Fas knockout indicates that Fas must play a role in killing
cells that don't pass positive selection (Krammer 2000). After positive
selection, thymocytes must undergo negative selection, at which point the cells
that are extremely autoreactive are killed. At first, scientists did not
think that Fas played a major role in negative selection because T cell receptors
in lpr mice are not excessively autoreactive (Kimura and Matsuzawa 1994).
But in fact the process is Fas independent only at low antigen concentrations.
lpr mice were capable of deleting highly reactive cells in the presence of small
amounts of antigen but the cells survived in the presence of high antigen concentrations.
Normal mice deleted reactive T cells at all antigen concentrations (Kishimoto,
Surh, and Sprent 1998).
The enlarged spleen and lymph nodes associated with the Fas mutation
occurs as a result of the accumulation and survival of lymphocytes after an
immune response. The ways that Fas causes T cell apoptosis are better
understood than the method of B cell apoptosis. Normally, activated T
cells proliferate in response to IL-2, which is required for clonal expansion.
But IL-2 also sensitizes T cells to Fas, making it easier for them to be targeted
after the pathogen has been removed. There is also evidence that activation
stimulates T cells to secrete FasL, which acts on the secreting cell and nearby
sensitive cells expressing Fas. In this way, activation regulates itself.
Without Fas, the signal to proliferate eventually turns off but the signal to
die is never received. Surviving effector cells have a high propensity
towards becoming self-reactive and may be the source of autoimmune symptoms
(Krammer 2000).
There is a lot of controversy in the scientific community about
the role of Fas in immune privilege. Some evidence has shown that Fas-expressing
immune cells that enter the testis and retina are killed in a FasL-dependent
process (Griffith et al. 1995), but other data suggest that FasL expression
promotes inflammation and tissue rejection. It is not known whether lpr
mice lack the ability to kill off T cells in the testis. Testis expressing
FasL survived indefinitely after transplantation into normal mice (Bellgrau
et al. 1995), but testis from gld mice (which do not produce FasL) were
quickly rejected. Other research shows that FasL activates a granulocyte
response that speeds the rejection process (Allison et al. 1997).
Research in this area is extremely applicable to specific immune suppression
during organ transplantation and cancer research. Many cancers express
FasL, which prevents active T cells from attacking the tumor. If we can
discover what factors influence whether FasL induces rejection or immune privilege,
it may develop into a powerful mechanism for targeting cancer and protecting
transplants (Maher et al. 2002).
Click here for the Autoimmune
lymphoproliferative syndrome (ALPS) homepage.
Drugs
With the exception of Fas-specific antibodies and the FasL, few
drugs have been created that bind specifically to the Fas molecule. There
are several drugs and substances that interact with the Fas signaling pathway.
Some of them, including FLICE, Bcl-2, and Bcl-XL have been described
above. Non-steroidal anti-inflammatory (NSAID) drugs have also been shown
to initiate apoptosis through the Fas signaling mechanism, and may be useful
in leukemia treatment. NSAIDs also kill colon epithelial cancer cells
through an unrelated pathway (Han et al. 2001). There is much anticipation
about application of apoptosis research to cancer therapy, transplant rejection,
and even AIDS.
References:
Allison J, Georgiou HM, Strasser A, Vaux DL. Transgenic expression
of CD95 ligand on islet beta cells induces a granulocytic infiltration but does
not confer immune privilege upon islet allografts. Proc Natl Acad Sci USA 1997
June 10;94(12):5986-90.
Bellgrau D, Gold D, Selawry H, Moore J, Fronzusoff Duke RC. A
role for CD95 ligand in preventing graft rejection. Science 1995 Nov 17;270
(5239):1189-92.
Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease
CPP32 by cytotoxic T-cell-derived granzyme B. Nature 1995 Oct 5;377(6548):446-8.
Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced
apoptosis as a mechanism of immune privilege. Science 1995 Nov 17;270(5239):1189-92.
Han Z, Pantazis P, Wyche JH, Kouttab N, Kidd VJ, Hendrickson EA. A Fas-associated
death domain protein-dependent mechanism mediates the apoptotic action of non-steroidal
anti-inflammatory drugs in the human leukemic Jurkat cell line. J Biol Chem
2001 Oct 19;276(42):38748-54.
Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and
mutagenesis of the Fas (Apo-1/CD95) death domain. Nature1996 Dec; 384(6610):638-41.
Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A,
Yoshiyuki S, Nagata S. The polypeptide encoded by the cDNA for human cell surface
antigen Fas can mediate apoptosis. Cell 1991 Jul 26; 66(2):233-243.
Ju ST, Matsui K, Ozdemirli M. Molecular and cellular mechanisms regulating
T and B cell apoptosis through Fas/FasL interaction. Int Rev Immunol 1999;18(5-6):485-513.
Kimura M, Matsuzawa A. Autoimmunity in mice bearing lprcg: a novel mutant gene.
Int Rev Immunol 1994;11(3):193-210.
Krammer PH. CD95's deadly mission in the immune system. Nature 2000 Oct 12;
407(6805):789-95.
Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes
in vivo. J Exp Med 1998 May 4;187(9):1427-38.
Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death:
The controversial role of Fas and Fas ligand in immune privilege and tumour
counterattack. Immunol Cell Biol 2002; 80(2):131-137.
Martin DA, Zheng L, Sieggel RM, Huang B, Fisher GH, Wang J, Jackson CE, Puck
JM, Dale J, Straus SE, Peter ME, Krammer PH, Fesik S, Lenardo MJ. Defective
CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative
syndrome, type Ia. Proc Natl Acad Sci USA 1999 Apr 13;96(8):4552-7.
Nagata S. Apoptosis by death factor. Cell 1997 Feb 7; 88(3):355-65.
Nagata S, Golstein P. The Fas death factor. Science 1995 Mar 10;267(5203):1449-56.
Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol
2002 Jul:2(7):527-35.
Davidson
College Immunology
taught by Dr.
Malcolm Campbell



 Fas
(also referred to as CD95 or APO-1) is a 48-kDa (319 amino acids) member of
the TNF/nerve growth factor receptor family. It has three regions: a N-terminal
extracellular domain, a single transmembrane domain, and a cytoplasmic domain.
The extracellular domain has three repeats of a cysteine-rich subdomain common
to all members of the TNF receptor family. The extracellular domain also
contains two glycosylation sites where sugars probably bind. The transmembrane
domain is 17 amino acids long (Itoh et al. 1991). Within the cytoplasm
lies an abundantly charged region known as the death domain. Figures 1
and 2 (click to see enlarged images
and figure legend) show models of the Fas death domain. The structure
consists of six antiparallel a helices capable
of self-association. This capability is extremely important to internal
cell signaling following Fas binding, as you will see below.
Fas
(also referred to as CD95 or APO-1) is a 48-kDa (319 amino acids) member of
the TNF/nerve growth factor receptor family. It has three regions: a N-terminal
extracellular domain, a single transmembrane domain, and a cytoplasmic domain.
The extracellular domain has three repeats of a cysteine-rich subdomain common
to all members of the TNF receptor family. The extracellular domain also
contains two glycosylation sites where sugars probably bind. The transmembrane
domain is 17 amino acids long (Itoh et al. 1991). Within the cytoplasm
lies an abundantly charged region known as the death domain. Figures 1
and 2 (click to see enlarged images
and figure legend) show models of the Fas death domain. The structure
consists of six antiparallel a helices capable
of self-association. This capability is extremely important to internal
cell signaling following Fas binding, as you will see below.