This web page was produced as an assignment for an undergraduate
course at Davidson College.
Mixed
Cryoglobulinemia
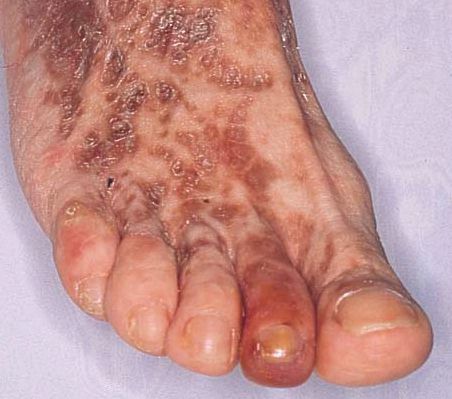 This
image shows the foot of a patient that has been suffering from mixed
cryoglobulinemia for 12 years. Purpura (rash due to blood vessel damage)
and the discoloration caused by chronic inflammation is clearly visible.
Gangrene infection is visible on the second toe. This image was obtained
from the Online Archives of Rheumatology
and permission has been requested. Click on the image to see the page from
which it was obtained.
This
image shows the foot of a patient that has been suffering from mixed
cryoglobulinemia for 12 years. Purpura (rash due to blood vessel damage)
and the discoloration caused by chronic inflammation is clearly visible.
Gangrene infection is visible on the second toe. This image was obtained
from the Online Archives of Rheumatology
and permission has been requested. Click on the image to see the page from
which it was obtained.
Mixed
cryoglobulinemia (MC) is a chronic autoimmune disorder that is almost always
associated with chronic liver inflammation due to hepatitis C virus (HCV)
infection. It is a combination of an immune complex disorder and a
lymphoproliferative disorder, with distinct symptoms caused by each aspect of
the disease. MC is believed to develop when chronic liver inflammation
causes B cells to grow out of control and produce excessive amounts of
antibodies, especially anti-IgG antibodies known as rheumatoid factors.
The B-1 (CD5+) cells are believed to be important in the production
of rheumatoid factors found in MC patients (Newkirk 2002). MC is a
relatively rare disorder, affecting only 13-54% of HCV infected
individuals. Despite its rarity, it is of significant interest because it
is one of the only autoimmune disorders so clearly associated with viral
infection (Della Rossa et al. 2001). The most effective current
treatments are antiviral medications such as IFN-a
(Gross 1999).
Symptoms
of Mixed Cryoglobulinemia
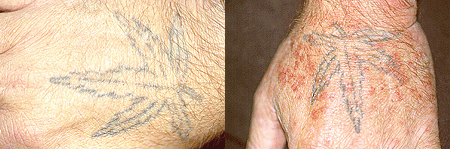
The
above image shows one of the less severe symptoms of MC, the purplish rash known
as purpura. The image was obtained from the Johns Hopkins Vasculitis
Center webpage and permission has been requested. Click on the image to
view the site from which it was obtained.
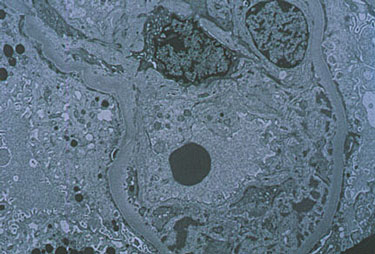
The above image
shows the electon microscopy image of the kidney of a patient with MC. The
image was obtained from the Johns Hopkins Vasculitis Center webpage and
permission has been requested. Click on the image to view the site from
which it was obtained.
The most common symptom of mixed cryoglobulinemia (MC) is purpura, which
is a purple spotted rash caused by internal bleeding. It is predominantly found in the extremities and is present
in over 90% of patients. The other
two extremely common symptoms are weakness (experienced by 89% of patients) and
joint pain (experienced by 83% of patients).
More serious but rare disorders include peripheral nerve damage, liver
damage, kidney damage (membranoproliferative glomerulonephritis due to
accumulation of immune complexes), and skin ulcers. The disease can also lead to B cell non-Hodgkin’s lymphoma
and hepatocellular carcinoma, which are found in only 7.5% and 2.4% of patients,
respectively (Ferri, Zignego, and Pileri 2002).
The most important laboratory indicators of MC are the immune complexes
of rheumatoid factor and polyclonal IgG immunoglobulin that precipitate out of
an affected patient’s cooled blood serum (Ferri, Zignego, and Pileri 2002).
Rheumatoid factors are autoantibodies that bind to the Fc region of IgG
(Newkirk 2002). The IgG molecules
of the immune complex are often specific for hepatitis C viral antigens. These
antigens precipitate out of solution as part of the immune complex (Agnello
1995). The two molecules interact
with a low affinity at body temperature (37 degrees Celcius).
As the temperature decreases, strengthening chemical interactions cause
the complexes to increase in size until they precipitate out of solution at 4
degrees Celcius. MC is also indicated by abnormally low levels of
complement in the blood (Ferri, Zignego, and Pileri 2002).
Risk
Factors
The most important risk factor for mixed cryoglobulinemia (MC) is
hepatitis C virus (HCV) infection. Anti-HCV
antibodies are present in 70 to 100% of patients with MC.
Nevertheless, only 13-54% of HCV infected patients develop the disorder
(Della Rossa et al. 2001). Women
are more likely than men to develop the disease: Women account for 63% of all
HCV-associated MC and 71% of type II MC (types I, II, and II will be
distinguished in the next section). HCV
infected patients carrying the HLA DR11 allele are more likely to develop MC,
whereas patients carrying the HLA DR7 allele seem to be protected against
developing MC (Newkirk 2002). Also,
there is evidence that other environmental factors may play a role.
MC is most prevalent in southern Europe and its frequency diminishes in
more northern locations. Its worldwide prevalence is not known (Ferri, Zignego and Pileri
2002).
Disease
Development
The exact mechanism by which MC arises from HCV infection is unknown.
MC symptoms fall into two distinct categories: those due to the
accumulation of immune complexes (membranoproliferative glomerulonephritis,
purpura, etc.) and those due to excessive lymphoproliferation (liver damage,
lymphomas, etc.). Immune complexes
accumulate because of the excessive amounts of IgG antibodies and rheumatoid
factors in the blood due to chronic inflammation. The IgG and rheumatoid
factors form immune complexes capable of activating complement (Newkirk
2002). Lymphoproliferation may be caused by a variety of factors,
including surface proteins on HCV that stimulate B cell activation, molecular
mimicry, and natural genetic mutations that become more likely with increasing
cell division. In many ways the immune complex disorder is a result of the
excessive lymphoproliferation, but the two aspects of the disease can become
self-sustaining (Ferri, Zignego, and Pileri 2002).
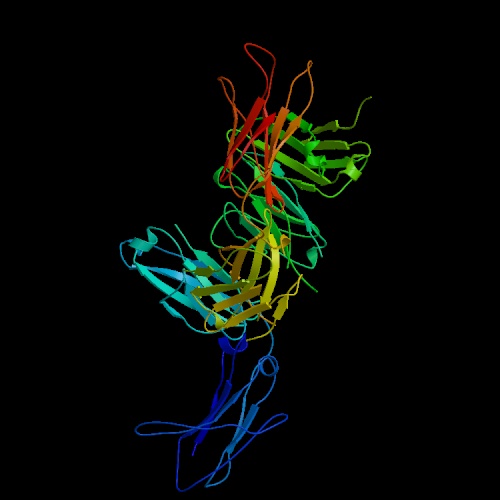 Rheumatoid factors (RFs) are antibodies against the Fc (constant region) of
IgG antibodies. An image of an RF fab fragment bound to an IgG Fc region
is at the left. Click on the image to go to the protein data bank site where
I got it. RFs are usually of the IgM isotype, especially in MC, but can be of
any isotype. RFs are produced by a subset of B cells known as B-1 or CD5
B cells. CD5 normally downregulates autoantibody production, and the pathology
found in MC may be due in part to defective CD5 signalling. B-1 cells
are found in the germinal centers that form in the liver as a result of HCV
infection and the chemical environment in these centers probably contributes
to the affinity and production levels of RFs in MC. The chemical environment
will include the cytokines secreted by Th1 or Th2 cells that have been selected
to fight the infection as well as gender-biased hormones. These hormones
may explain the high incidence of MC in women as opposed to men. The normal
role of RFs in immune response is unclear, but they are produced transiently
in response to almost all infectious agents. Their main role is probably
to aid in immune complex clearance by making complexes larger and activating
complement. In MC, they are a major component of the cryoglobulins that
precipitate out of patients blood serum, an important laboratory indicator of
the disease (Newkirk 2002).
Rheumatoid factors (RFs) are antibodies against the Fc (constant region) of
IgG antibodies. An image of an RF fab fragment bound to an IgG Fc region
is at the left. Click on the image to go to the protein data bank site where
I got it. RFs are usually of the IgM isotype, especially in MC, but can be of
any isotype. RFs are produced by a subset of B cells known as B-1 or CD5
B cells. CD5 normally downregulates autoantibody production, and the pathology
found in MC may be due in part to defective CD5 signalling. B-1 cells
are found in the germinal centers that form in the liver as a result of HCV
infection and the chemical environment in these centers probably contributes
to the affinity and production levels of RFs in MC. The chemical environment
will include the cytokines secreted by Th1 or Th2 cells that have been selected
to fight the infection as well as gender-biased hormones. These hormones
may explain the high incidence of MC in women as opposed to men. The normal
role of RFs in immune response is unclear, but they are produced transiently
in response to almost all infectious agents. Their main role is probably
to aid in immune complex clearance by making complexes larger and activating
complement. In MC, they are a major component of the cryoglobulins that
precipitate out of patients blood serum, an important laboratory indicator of
the disease (Newkirk 2002).
Cryoglobulins are classified into three groups. Type I cryoglobulins are
composed of only monoclonal antibodies and are found mostly in patients with
lymphoid tumors. Type II cryoglobulins are composed of a mix of polyclonal
IgG and monoclonal IgM RFs. Type III are polyclonal IgG and IgM RFs.
Types II and III are classified as mixed cryoglobulinemia and can both be
produced as a result of infectious agents (Ferri, Zignego, and Pileri
2002). Immune complexes in the blood can increase blood viscosity and
accumulate in the nephron and capillaries to cause membranoproliferative
glomerulonephritis and purpura (Della Rossa et al. 2001).
Lymphoproliferation is responsible for liver damage (due to chronically inflamed
germinal centers), cancers, and possibly nerve damage in MC patients.
The mechanism by which HCV may cause lymphoproliferation is still being researched,
but there are several theories. The first is based on the finding that
the HCV protein E2 binds CD81, which is part of the B cell coreceptor
along with CD21 and CD19. This theory argues that E2-CD81 binding reduces
the threshold necessary for B cell stimulation. The threshold reduction results
in an overreactive B cell that could produce excessive levels of antibody and
proliferate. Since B-1 cells, which are implicated in RF production, are
thymus independent, this theory seems especially plausible for explaining excessive
levels of RFs in MC patients. The second theory argues that HCV activates
B cells specific for antibody:HCV protein complexes that are constantly available
due to the chronic infection. These B cells would be constantly stimulated
to divide while overproducing antibodies. The final theory is that the
prolonged division cycle induced by chronic infection naturally increases the
likelihood that mutations will occur that may produce excessive proliferation
(Ferri, Zignego, and Pileri 2002).
Treatment
The most
primary goal of any treatment regimen for MC is elimination of the HCV infection.
When the level of virus in the serum decreases, patients usually experience
a decrease in symptoms as well. For this reason, Interferon-a
is the current drug of choice (Gross 1999). Interferon-a
is a general antiviral treatment that prevents viral replication within cells,
increases antigen presentation, and activates NK cells to clear out infection
(Janeway et al. 2001). Unfortunately, interferon treatment sometimes
only produces transient results and has been shown to increase the likelihood
of peripheral sensory neuropathy. These effects can be reduced by a combination
the interferon-a treatment with ribavirin, although
the efficacy of this combination has not yet been proven. Studies into HCV vaccines
have demonstrated the feasibility of creating antibodies that block HCV binding
sites. This treatment would prevent them from infecting new cells (Ferri, Zignego,
and Pileri 2002).
The secondary goal
of MC treatment is alleviation of the symptoms, which requires immunosuppression.
Non-steroidal anti-inflammatory drugs are used to treat minor symptoms such
as purpura, weakness, and joint pain. Steroids are prescribed for more serious
symptoms such as sensory neuropathy and glomerulonephritis. A low-antigen content
diet has also shown promise for treatment of liver disease. It is thought that
the diet works by decreasing the amounts of large ingested substances that compete
with immune complexes for clearance by the mononuclear phagocytic system. If
the diet reduces the concentration of these substances, the immune complexes
may be more efficiently cleared. The most seriously life threatening conditions,
such as acute progressive glomerulonephritis, motor neuropathy, and hyperviscosity
syndrome, are treated with plasma exchange therapy accompanied by immunosuppressive
drugs. This treatment rapidly decreases the number of circulating immune complexes
(Della Rossa et al. 2001).
Sources
Agnello V. The Aetiology of Mixed
Cryoglobulinaemia Associated with Hepatitis C Virus Infection. Scand J Immunol
1995;42:179-185.
Della Rossa A, Tavoni A, Baldini
C, Bombardieri S. Mixed Cryoglobulinemia and Hepatitis C Virus Association: Ten
Years Later. IMAJ 2001 June;3:430-434.
Ferri C, Zignego AL, Pileri SA.
Cryoglobulins. J Clin Pathol 2002; 55:4-13.
Gross WL. New Concepts in treatment
protocols for severe systemic vasculitis. Curr Opin Rheumatol 1999; 11:41-46.
Janeway, CA Jr, Travers P, Walport
M, Shlomchik MJ. Immonobiology 5. New York: Garland Publishing, 2001.
Newkirk MM. Rheumatoid Factors:
Host Resistance or Autoimmunity? Clinical Immunology 2002 Jul;104(1):1-13.
Davidson
College Immunology
taught by Dr.
Malcolm Campbell


 This
image shows the foot of a patient that has been suffering from mixed
cryoglobulinemia for 12 years. Purpura (rash due to blood vessel damage)
and the discoloration caused by chronic inflammation is clearly visible.
Gangrene infection is visible on the second toe. This image was obtained
from the Online Archives of Rheumatology
and permission has been requested. Click on the image to see the page from
which it was obtained.
This
image shows the foot of a patient that has been suffering from mixed
cryoglobulinemia for 12 years. Purpura (rash due to blood vessel damage)
and the discoloration caused by chronic inflammation is clearly visible.
Gangrene infection is visible on the second toe. This image was obtained
from the Online Archives of Rheumatology
and permission has been requested. Click on the image to see the page from
which it was obtained.


