This web page was produced as part of an undergraduate assignment at Davidson College.
Introduction to TdT. Terminal deoxynucleotidyl transferase is an enzyme which is located in the nucleus of immature lymphocytes (UW, Laboratory Medicine 1999). It is a DNA polymerase able to act without template direction (Landreth et al. 1981). In a study by John Hansen, the general structure of TdT was determined using a rainbow trout thymic clone encoding TdT. The clone was 2.3 kilobase and had an open reading frame of 1,506 base pairs. Through sequence comparisons, it was shown that TdT was highly conserved. Four PKC phosphorylation sites were present and are thought to be involved in TdT regulation (Hansen 1997). Studies have also shown that there is a promoter region extending through nucleotides -111 to +58. It is believed that transcription of TdT is controlled by demethylation of this region (Nourrit et al. 1999). The exact structure of the enzyme is still being determined, and it has not yet been crystallized. TdT increases diversity of Ig and TCR by adding N-nucleotides to the rearrangement joints of B- and T-cell antigen receptors during development. Although TdT is expressed in lymphoid progenitors, rearrangement can occur without it, and it is not expressed when B and T lymphocytes first enter the peripheral immune system. In human B lymphocytes, TdT is expressed in the pro-B cell but not in the pre-B cell following heavy chain rearrangement. For this reason, TdT is found in only 25% of human light chains but approximately 100% of heavy chains. Early pro-B cells are located near the endosteum of the bone marrow and have a high concentration of TdT. As they progress through development, the cells lose TdT and eventually pass to the sinuses of the marrow to await export (Janeway et al. 1999).
Function of TdT. Diversity in both B and T lymphocytes is primarily a result of recombination (Figure. 1) (Schatz). This paper concenctrates on TdT function in Ig (B-cells). In heavy and light chain immunoglobulins, junctional diversity can further increase overall diversity by the addition of P- and N- nucleotides within the third hypervariable region (located between the V and J regions; partially encoded by D in heavy). P-nucleotide additions are the result of a hairpin intermediate in the course of recombination, while N-nucleotides (non-template-encoded) are added by TdT (Janeway et al. 1999). N additions serve to add codons to the heavy chain variable region CDR3 and may alter the reading frame of the D gene from RF1 to RF2 or -3. N additions also disrupt "homology directed" gene combinations which occur from repetition of recombination of the same genes (Molano et al. 2000). Following hairpin cleavage, TdT adds the nucleotides to the 3' ends of the coding regions (Schatz). TdT is capable of adding up to twenty nucleotides at which point in time the single-stranded ends of the two genes form base pairs. Repair enzymes are employed to remove non-matching bases, synthesize bases to fill in gaps, and ligate these bases to the P-nucleotides. Since TdT is expressed only for a relatively miniscule time period during heavy chain rearrangement in B-cells, N-nucleotides are not often seen in light chain genes (Janeway et al. 1999).

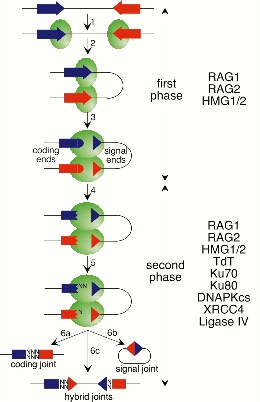
Model for V(D)J recombination. Shows that terminal deoxynucleotidyl transferase (TdT) can, if present, add nontemplated nucleotides (N) to the 3' ends of the coding regions (step 5). Model based on that proposed by Lewis and Gellert (Cell vol. 59:585-588) and taken from Sem. Immunol., vol. 9, 149-159 (1997) (Schatz). David Schatz, PhD, Yale University School of Medicine Permission to use this image has been requested. Image will be removed if permission refused.
Association of TdT with Ku. Ku is a heterodimeric protein that binds DNA ends. It consists of 70- and 86-kDa subunits. Ku is required for V(D)J recombination and DNA double-strand break (DSB) repair. It has been shown that TdT can react with each subunit of Ku and with the heterodimer. The N terminal region of TdT (131 aa) is required for this interaction and contains a BRCA1 C-terminal domain which mediates DNA repair. This suggests that the interactions of TdT with Ku serve to bring TdT to sites of DSB's so that repair can occur (Mahajan et al. 1999). Although rare, there is occasional deletion in the gene encoding the 86-kDa subunit of Ku within Ig and TCR coding joints. This, in homozygous organisms, leads to a very sparse population of N regions. In SCID mice with a shortened form of DNA-PK (catalytic subunit), there is a lower number of coding joints with N regions thus implying a connection between DNA-PK and the addition of N nucleotides. It has been shown TdT does form a complex with DNA-PK, and that DNA-PK can, in vitro, limit the composition as well as the length of TdT N nucleotide additions (Mickelson et al. 1999).
Further Regulation of TdT. N regions are scarce in Ig light chains, and TdT transcription is down-regulated following the pre-B cell IgM heavy chain production. It is possible that IgM heavy chain negatively regulates TdT expression. In an experiment, Wasserman et al. looked at TdT expression in normal mice, recombination-deficient mice with IgM transgenes, and in transformed pro-B cells lines transfected with IgM constructs. In the normal mice, they found TdT to be sharply down-regulated at the early pre-B cell level. The transgenes from the deficient mice behaved in a similar manner. Transfection also led to reduction of TdT expression. Together, these three observations suggest IgM production leads to a down-regulation of TdT (Wasserman, et al. 1997).
Effect of TdT Deficiency. A 1981 study by Landreth et al. suggested that lack of TdT+ cells resulted in the autoimmune disorder which leads to death within eight weeks of birth for motheaten mice (Landreth et al. 1981). Later experiments using other autoimmune disorders had different results. In 1997, a study by Weller et al., revealed that TdT is not essential for the development of B-cells expressing autoantibodies, but, due to polyreactivity, the number of these cells is lessened in TdT knockout mice (Weller, et al. 1997). When the TdT null mutation was introduced into the (NZB x NZW)F1 (B/W) mouse strain, it resulted in a reduction of autoimmune nephritis, or lupus nephritis. Although the TdT knockout mice did produce anti-ADN and anti-histone autoantibodies, there was no evidence of inflammation in the kidney. This supports the suggestion that TdT plays a role in the production of autoantibodies, and that inhibition of TdT may protect against autoimmunity (Conde et al. 1998). In a third study conducted by Molano et al., the TdT knockout genotype was bred onto the autoimmune C57BL/6-Fas(lpr) background in mice. The TdT-deficient mice showed lower levels of sera-DNA and rheumatoid factor activity, as well as shorter variable heavy chain CDR3 regions and fewer arginines. Lack of TdT resulted in limited Ig diversity which lessened production of anti-DNA antibodies and rheumatoid factors. This experiment shows that TdT is important for autoantibody production, pointing to the possibility of employing TdT inhibition to limit the production of autoantibodies and potentially eliminate certain autoimmune diseases (Molano et al. 2000).
TUNEL. This technique, TdT-mediated dUTP nick end-labeling assay, is employed to detect apoptotic cells (Zhang, et al. 1999). Using TdT with biotin-coupled uridine, it is possible to label 3' ends resulting from apoptosis. Streptavidin, which binds to biotin, is tagged with an enzyme and added to the tissue or culture. The colorless enzyme substrate will be reacted upon producing a colored precipitate in apoptotic cells (Janeway et al. 1999). Figure 2 shows the use of TUNEL staining on apoptotic kidney cells (Alabi et al. 1999).
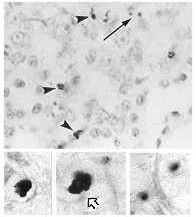
Top: Apoptotic cells visible in kidney cortical epithelium. TUNEL staining provides evidence of DNA fragmentation.
Bottom: Details. DNA fragmentation visible in cytoplasm (right) and evidenced by clumping of nuclear material (left, center).
These are pictures from El Mouedden, et
al. 2000 "Apoptosis
in renal proximal tubules of rats treated with low
doses of aminoglycosides", published in Antimicrobial Agents
and Chemotherapy, vol. 44, pages 665-675.
Taken from: Alabi et al., 1999 (modified) Permission obtained.
TdT's Role in Medicine. The nuclei of precursor lymphoid neoplasms (lymphoblastic leukemia, lymphoma) contain TdT. It is possible to use TdT as a marker for diagnosis of these diseases. Immunoflourescence (IF) and flow cytometry are available methods for detecting and assaying the presence of TdT (UW, Laboratory Medicine 1999). In one example of the use of IF, the IgG fraction of anti-TdT serum was mixed with flouresceinisothiocyanate and used on fixed bone marrow/blood smears. IF allows the TdT to be localized within the cell and correlated to specific cell populations. 64% of cases of acute lymphoblastic leukemia (ALL) were found to be TdT+ thus corresponding to the theory that high levels of TdT in the nucleus suggest the presence of ALL (Nair et al. 1987). In 1995, a study was done to confirm the diagnostic value of TdT in detecting acute leukemias. Previous methods of detecting TdT, including IF, had recently been questioned. This study used flow cytometry to quantitatively analyze the results. Out of 58 cases of acute leukemia, the highest levels of TdT expression were associated with ALL and the lowest with acute myeloid leukemia (AML). Flow cytometry allows greater distinction to be made between ALL and AML and is better at defining stages of maturation within T-ALL and pre-B ALL. Through the use of flow cytometry, TdT has retained its importance as a diagnostic tool in acute leukemias (Farahat et al. 1995). According to Healthgate.com, high or increased levels of TdT can, through the TdT test, indicate acute lymphocytic anemia, the blastic phase of chronic myelogenous leukemia, lymphoblastic leukemia, or leukemia. Normal TdT levels in the blood range from 0 to 10IU/1013 cells (Healthgate.com TdT test 1999).
Future Research. John Kearney, PhD, at the University of Alabama Birmingham, is currently working on determining the impact of TdT expression on CDR3 region diversity and its effects on fetal, perinatal, and adult B-cells. Kearney is using transgenic mice to introduce N region additions in different stages of B-cell development with the goal of determining the differentiative events in early development that lead to the eventual B-cell repertoire. Since neonates normally do not have TdT present in their cells, Kearney will attempt to determine whether this restriction is more important than eventual adult diversity or not and whether neonatal restriction is necessary for the development of a fully functional immune system. His ultimate aim is to reach an understanding of fetal and neonatal B-cell roles in establishing the immune system and in autoimmune diseases, B-cell neoplasia, immunodeficiency diseases, and the establishment of better vaccines (John Kearney, PhD UAB Microbiology).
SOURCES.
Alabi A, El Mouedden M, Gerbaux C, Leys K, Mingeot-Leclercq MP, Tyteca D, Tulkens PM, Van Bambeke F. 1999 December 26. Cellular toxicity of antibiotics and other drugs. <http://www.md.ucl.ac.be/entites/farm/facm/cellular_toxicity.htm>. Accessed 2000 March 2.
Conde C, Weller S, Gilfillan S, Marcellin L, Martin T, Pasquali JL. 1998 Dec 15. Terminal deoxynucleotidyl transferase deficiency reduces the incidence of autoimmune nephritis in (New Zealand Black x New Zealand White)F1 mice. Journal of Immunology 161(12):7023-30.
El Mouedden M, et al. 2000. Apoptosis in renal proximal tubules of rats treated with low doses of aminoglycosides. Antimicrobial Agents and Chemotherapy 44:665-675.
Farahat N, Lens D, Morilla R, Matutes E, Catovsky D. 1995 Apr. Differrential TdT expression in acute leukemia by flow cytometry: a quantitative study. Leukemia 9(4):583-7.
Hansen JD. 1997. Characterization of rainbow trout terminal deoxynucleotidyl transferase structure and expression. TdT and RAG1 coexpression define the trout primary lymphoid tissues [abstract]. In Immunogenetics 46(5):367-75. <http://link.springer-ny.com/link/service/journals/00251/bibs/7046005/70460367.htm>. Accessed 2000 March 1.
Healthgate.com. 1999. Terminal deoxynucleotidyl transferase test. <http://www.healthgate.com/tests/test328.shtml>. Accessed 2000 March 2.
Janeway CA, Travers P, Walport M, Capra JD. 1999. Immunobiology: the immune system in health and disease. New York, NY: Elsevier Science Ltd/Garland Publishing. P.97-8,187,198,202.
Kearney JF. Homepage. <http://www.microbio.uab.edu/faculty/kearney-j.htm>. Accessed 2000 March 2.
Landreth KS, McCoy K, Clagett J, Bollum FJ, Rosse C. 1981 Apr 2. Deficiency in cells expressing terminal transferase in autoimmune (motheaten) mice. Nature 290(5805):409-11.
Mahajan KN, Gangi-Peterson L, Sorscher DH, Wang J, Gathy KN, Mahajan NP, Reeves WH, Mitchell BS. 1999 Nov 23. Association of terminal deoxynucleotidyl transferase with Ku. Proceedings of the National Academy of Science USA 96(24):13926-31.
Mickelson S, Snyder C, Trujillo K, Bogue M, Roth DB, Meek K. 1999 July 15. Modulation of terminal deoxynucleotidyl transferase activity by the DNA-dependent protein kinase. Journal of Immunology 163(2):834-43.
Molano ID, Wloch MK, Alexander AA, Watanabe H, Gilkeson GS. 2000 Jan. Effect of a genetic deficiency of terminal deoxynucleotidyl transferase on autoantibody production by C57BL6Fas(lpr) mice. Clinical Immunology 94(1):24-32.
Nair CN, Gothoskar BP, Gladstone B, Badrinath Y, Chhajlani V, Karnik M, Nerurkar A, Damle SR, Redkar SL, Gopal R, et al. 1987. Terminal deoxynucleotidyl transferase in acute leukemias by direct immunoflourescence. Neoplasma 34(3):305-11.
Nouritt F, Coquilleau I, D'Andon MF, Rougeon F, Doyen N. 1999 Sep 17. Methylation of the promoter region may be involved in tissue-specific expression of the mouse terminal deoxynucleotidyl transferase gene. Journal of Molecular Biology 292(2):217-27.
Schatz D. no date. Homepage. <http://info.med.yale.edu/immuno/fac_schatz.html>. Accessed 2000 February 29.
University of Washington, Department of Laboratory Medicine. 1999 May 10. Immunohistochemistry. <http://depts.washington.edu/labweb/Division/Hematology/ihc.html>. Accessed 2000 March 1.
Wasserman R, Li YS, Hardy RR. 1997 Feb 1. Down-regulation of terminal deoxynucleotidyl transferase by Ig heavy chain in B lineage cells. Journal of Immunology 158(3):1133-8.
Weller S, Conde C, Knapp AM, Levallois H, Gilfillan S, Pasquali JL, Martin T. 1997 Oct 15. Autoantibodies in mice lacking terminal deoxynucleotidyl transferase: evidence for a role of N region addition in the polyreactivity and in the affinities of anti-DNA antibodies. Journal of Immunology 159(8):3890-8.
Zhang P, Duchambon P, Gogusev J, Nabarra B, Sarfati E, Bourdeau A, Drueke TB. 2000 Feb. Apoptosis in parathyroid hyperplasia of patients with primary of secondary uremic hyperparathyroidism. Kidney International 57(2):437-45.
Go back to Heather's Immunology Home Page
Learn more about Immunology from the Davidson College Immunology Home Page
©Copyright 2000 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: hebaker@davidson.edu