This web page was produced as an assignment for an undergraduate
course at Davidson College.
INTERLEUKIN-12
Aliases:
AKA: T-cell stimulating factor (TSF), natural-killer cell stimulatory
factor (NKSF), and cytotoxic lymphocyte maturation factor (CLMF).
Introduction:
Interleukin 12 (IL-12) is an important regulatory cytokine that has
a function central to the initiation and regulation of cellular immune
responses. It has the capacity to regulate the differentiation of
naive T cells into TH1 cells, which is crucial in determining resistance
and the type of response that will be elicited in response to a particular
pathogen. It stimulates the growth and function of T cells and alters
the normal cycle of apoptotic cell death.
Structure and location of IL-12:
IL-12 is one of a large group of cytokines that folds into a bundle
of four alpha-helices. It is a heterodimer of 70kDa that is composed
of two disulfide-linked subunits, of mass 35kDa and 40kDa. These
subunits are coded by different, and seemingly unrelated genes (Brandhuber
et al., 1987). Only a single receptor chain has been identified for
IL-12, labeled the IL-12Rbeta1 receptor. Itís structure of about
100kDa in humans and mice is most homologous to the leukemia inhibitory
factor (LIF) receptor (Chua et al., 1995). IL-12 p40 and p35 chains
are encoded by two separate genes that bear no apparent homology.
The gene encoding the p40 chain is mapped to chromosome 5q31-q33, a region
that encodes many cytokines and cytokine receptors, and the gene encoding
the p35 chain is located on chromosome 3p12-3q13.2. The two genes
that encode the murine p40 and p35 counterpart chains contain 70 and 60%
sequence homology, respectively, to the human genes (Xiaojing et al., 1996)
Detection of IL-12 Gene Expression:
There are three methods to detect the cellular expression of IL-12.
They are: Northern blot, RNAse protection, and competitive/quantitative
PCR. Polyclonal antibodies to the p40 and p35 chains are also
formed to detect the presence of IL-12. The production of IL-12 requires
an apparent coordinated expression of both p40 and p35 chains that makes
the formation somewhat challenging. Additionally, the p40 homodimer
has been shown to have an antagonistic role in the mouse (Gillessen, 1995).
Furthermore, the p35 chain of IL-12 has a more widespread expression throughout
the individual and its mRNA was found in both brain and lung tissue, while
the p40 chain was not detected in either of these areas. It is thought
that the p35 chain may have a function within the body independent of its
regulatory capacity through IL-12. Upon activation of phagocytic
cells, accumulation of IL-12 is observed to be somewhat delayed in relation
to the expression of other inflammatory cytokines, sich as TNF-alpha and
IL-1beta, and then subsides after several hours. The activation of
the p40 chain requires active protein synthesis, mainly at the initiation
of the stimulus. The production of IFN-gamma has a very powerful
effect in enhancing the ability of phagocytic cells to produce IL-12 and
IL-12 also enhances the production of IFN-gamma, creating a positive reinforcement
loop. Early induction by IL-12 of IFN-gamma expression is key to
the initiation of the innate immune response.
Medical Uses:
IL-12 has great potential as a vaccine adjuvant for promoting cell-mediated
immunity and a TH1 cell response. Not only does immunization with
IL-12 as adjuvant promote a long-term and stable TH1 response, it also
enhances the primary TH1 response when given in conjunction with other
adjuvants. However, O'Garra notes that repeated exposeure to antigen
and IL-12 is necessary to establish a stable TH1 response (O'Garra et al.,
1996).
Deletion of genes that form IL-12 in gene knockout mice:
Cytokine responses were monitored for IL-12 knockout mice in vivo to
further define the important role of the differentiation of naive T cells
into TH1 cells. This differentiation helps create a balance between
the cell-mediated and humoral immunity. In this experiment, the knockout
mice were observed to be completely viable and fertile, and displayed no
developmental abnormalities. However, on an immunological level,
these mice were noticed to have a reduced capacity to cause a TH1 cell
response and also a relative inability to produce IFN-gamma in response
to toxins engulfed by phagocytic cells (Magram, et al., 1996).
Function of Co-receptors in the action of IL-12 in mice:
Activated T cells were injected into the mice and the mice were tested
for antibodies that might be responsible for the regulation of T-cell responsiveness
to the binding of IL-12. CD2 was identified as one of these regulators
along with its major ligand, CD58, which binds to its adhesion portion
and effectively inhibits the response of the T cells to bound IL-12.
However, this regulation does not effect the binding of IL-12 in any way.
In fact, the presentation of adhesion molecules by an antigen presenting
cell, a monocyte for example, illustrates how these APCís can regulate
the response of T cells to a cytokine, without disturbing the cytokine
binding interaction (Gollob and Ritz, 1996).
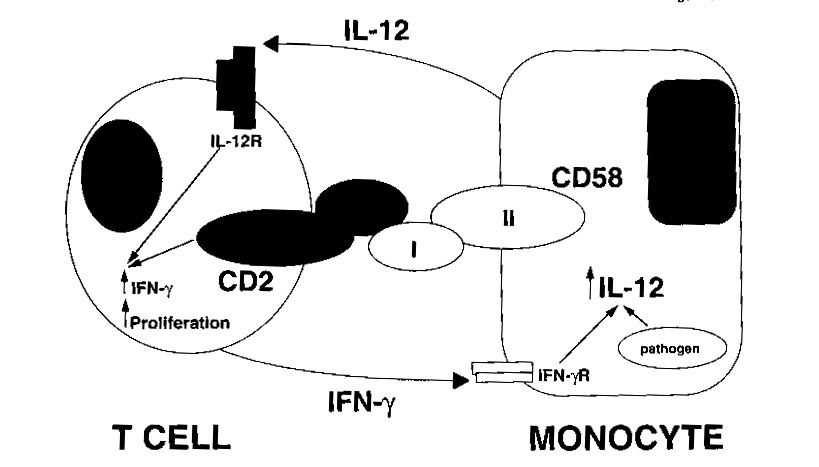
Figure 1: This shows how the binding of the CD-2 ligand, CD-58, is
a very important part of the positive reinforcement of the T-cell by the
monocyte (Gollob and Ritz, 1996). This interaction causes the production
of IFN-gamma by IL-12. Permission is pending from the author of this
article for the use of this figure. If not approved for use, it will
be removed from the site.
Normal Function/Signal Transduction involving IL-12:
The induction of a cell-mediated immune response to a specific antigen
is regulated, for the most part, by the release of cytokines. IL-12
is produced by macrophages, monocytes, dendritic cells, and B cells in
response to bacterial products and intracellular parasites. The biological
effects of the production of IL-12 are directed at T cells and NK cells.
IL-12 is responsible primarily for the subsequent production of IFN-gamma
and tumor necrosis factor-alpha (TNF-a) from both NK cells and helper T
cells. Researchers have concluded in recent experiments that because
IL-12 is responsible for the production of IFN-gamma, itís immunological
action must be directed primarily to those cells that are capable of producing
IFN-g. The cells that produce IFN-g most often are those activated
T cells that also have the coreceptor, CD30, present on their surface.
Therefore, CD30+ T cells are a target of the actions of IL-12 (Alzona,
1994). IL-12 also stimulates the rate at which NK cells and helper
T cells proliferate following antigen activation. In addition, the
lytic capacities of both NK and helper T cells are increased by the presence
of IL-12. IL-12 has the specialized function of leading naive CD4+
T cells to differentiate toward the TH1 cell type in order to prepare for
the release of IFN-gamma and for the development of the cell-mediated immune
response (Hsieh, 1993). However, IL-12 is not effective in the down
regulation by means of reversing TH2 cells differentiation. IL-12
and IL-2 are both important cytokines in the regulation of a cell-mediated
immune response, IL-2 being responsible for stimulating the growth and
proliferation of T cells, while IL-12 stimulates the differentiation of
the CD4+ T cells into TH1 cells. The sites of phosphorylation and
activation of particular transcription factors during the signaling pathways
of these two cytokines are not completely understood and a likely model
based on experimental data is shown below.
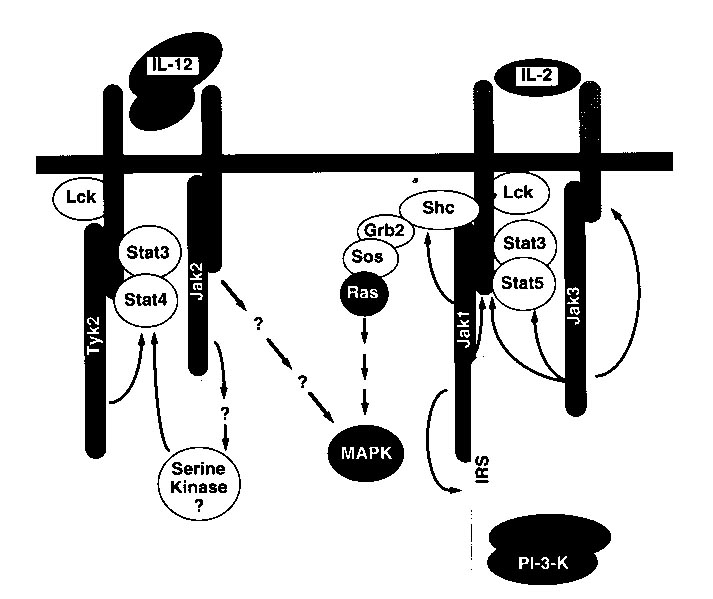
Figure 2: A model produced from experimental data, indicating the possible
signaling pathways for two similar cytokines, IL-12 and IL-2 (Bacon et
al., 1996). Permission is pending for this figure from the
author of the article. If permission is not granted for use of this
figure on this site, it will be removed.
It is also important to note the function that IL-12 has in the regulation
of the production of antibody isotypes. The direct binding of IL-12
to B cells causes a long-term enhancement of antibody production, in addition
to the isotype switching that is caused by the induction of IFN-g production
as IL-12 stimulates the differentiation of TH1 cells (Metzger 114).
References:
Alzona, M., H.M. Jack, R.I. Fisher, and T.M. Ellis. 1994. Journal
of Immunology. 153, 2862-2867.
Bacon, C.M., S.S. Cho, and J.J. O'Shea. 1996. Annals of the New
York Academy of Sciences. 795, 41-59.
Brandhuber, B.J., T. Boone, W.C. Kenney, and D.B. MacKay. 1987.
Science. 23, 1707-1709.
Chua, A.O, V.L. Wilkinson, D.H. Presky, and U. Gubler. 1995.
Journal of Immunology. 155, 4286-4294.
Gillessen, S., D. Carvajal, P. Ling, F.J. Podlaski, D.L. Stremlo, P.C.
Familletti, U. Gubler, D.H. Presky, A.S. STern, and M.K. Gately. 1995.
European Journal of Immunology. 25, 200-206.
Gollob, J.A. and J. Ritz. 1996. Annals of the New York Academy
of Sciences. 795, 71-81.
Hsieh, C.S., S.E. Macatonia, C.S. Tripp, S.F. Wolf, A. O'Garra, and
K.M. Murphy. 1993. Science. 260, 547-549.
Magram, J., J. Sfarra, S. Connaughton, D. Faherty, R. Warrier, D. Carvajal,
C Wu, C. Stewart, U. Sarmiento, and M. Gately. 1996. Annals of
the New York Academy of Sciences. 795, 60-70.
Metzger, D, J.M. Buchanan, J.T. Collins, T.L. Lester, K.S. Murray,
V.H. Van Cleave, L.A. Vogel, and W.A. Dunnick. 1996. Annals of
the New York Academy of Sciences. 795, 100-115.
O'Garra, A., B. E. Murphy, K. Shibuya, N. Hosken, P. Openshaw, V. Maino,
K. Davis, and K. Murphy. 1996. Reversibility of T helper 1 and 2
populations is lost after long-term stimulation. Journal of Exp. Medicine.
183, 901-913.
Xiaojing, M.A., Miguel Aste-Amezaga, and Giorgio Trinchieri. 1996.
Annals of the New York Academy of Sciences. 795, 13-25.
Return to Brent Wilson's Immunology Home Page
Link
to the Immunology Home Page
Link to the Biology Department
Home Page
Link to the Davidson College Home
Page
Send questions or comments to: brwilson@davidson.edu

