Interferon Gamma (IFN-g)

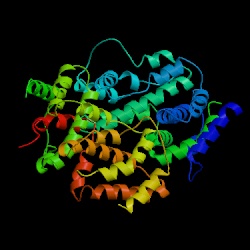
3D structure of human IFN-g
Image courtesy of Protein Data Bank http://www.rcsb.org/pdb/
Introduction:
What are interferons?
Interferons (IFN’s) are a group of proteins known primarily for their role in inhibiting viral infections and in stimulating the entire immune system to fight disease, in response to pathogens. Interferons belong to the large class of glycoproteins known as cytokines. In contrast to antibodies, interferons are not virus specific but host specific. Thus, viral infections of human cells are inhibited only by human interferon (Janeway et al., 2005).
The first interferon was discovered in 1957 by Alick Isaacs and Jean Lindenmann. During their investigation, the two scientists found that virus-infected cells secreted a special protein that caused both infected and non-infected cells to produce other proteins that prevented viruses from replicating. They named the protein interferon because it "interferes" with infection. Initially, scientists thought there was only one interferon protein, but subsequent research showed that there are different types of interferon proteins (Plotnikoff, 1999).
Types of interferons:
Interferons are classified primarily through their amino acid sequence. Interferon-alpha, -beta, -tau, and -omega, which have relatively similar amino acid sequences, are classified as type I interferons. Type I interferons are known primarily for their ability to make cells resistant to viral infections. Interferon gamma is the only type II interferon, classified as such because of its unique amino acid sequence (NCBI amino acid sequence). This interferon is known for its ability to regulate overall immune system functioning (Mitcham, 2005).
In addition to their structural makeup, type I and type II interferons have other differences. Type I interferons are produced by almost every cell in the body while the type II interferon-gamma is produced only by specialized cells in the immune system. The two classes also bind to different kinds of receptors, which lie on the surface of cells and attract and combine with specific molecules of various substances.
IFN-g:
Brief overview:
Interferon Gamma (IFN-g), also called immune or type II interferon is a pleiotropic
cytokine involved in the regulation of nearly all phases of immune and inflammatory
responses, including the activation, growth and differentiation of T-cells,
B-cells, macrophages, NK cells and other cell types such as endothelial cells
and fibroblasts. It enhances MHC expression on antigen-presenting cells, and
IFN- production is characteristic of Th1 differentiation (Romagnani, 1997).
It also has weak antiviral and anti-proliferative activity, and potentiates
the antiviral and anti-tumor effects of IFN- and IFN-beta. Defects in the IFN-g
activation pathway are associated with susceptibility to severe microbacterial
infections, and diseases such as multiple sclerosis (Seppa, 2000). In
contrast to type I interferons, IFN-g is held to be more important as an immunoregulator
than as an antiviral agent. It enhances the cytotoxic activity of T cells, macrophages
and natural killer cells and thus has antiproliferative effects. It also increases
the production of antibodies in response to antigens administered simultaneously
with alpha-interferon, possible by enhancing the antigen-presenting function
of macrophages (Mitcham, 2005).
Gene Structure:
IFN-g has been located on the human chromosome 12q15 (Bureau et al., 1995)3D structure:
INF-g is a homodimer glycoprotein formed by antiparallel association of two 21-24kD subunits. Each subunit has 6 alpha-helices held together by short nonhelical sequences. There are no ß-sheets. The subunits have a flattened elliptical shape, whereas the overall structure of the dimmer is globular (Ealick et al., 1991).
.................................
Fig 1a. IFN-g monomer.................................................................Fig 1b. INF-g dimer
Fig. 1 3D
structure of IFN-g: (a) The human interferon-g
protein is primarily a helical, with six helices in each subunit that
comprise approximately 62% of the structure; there is no b sheet. (b)
Interferon-g, which is dimeric in solution, also crystallizes with two
dimers related by a noncrystallographic twofold axis in the asymmetric
unit. The dimeric structure of human interferon-g is stabilized by the
intertwining of helices across the subunit interface with multiple intersubunit
interactions. This view is parallel to the dimer two-fold axis (Images
courtesy of the Cornell University Chemistry Department) |
Mode of action:
IFN-g is produced by Th0-cells, activated Th1-cells (CD4+) and cytotoxic T
cells (CD8+), and by NK cells. Practically any antigen can cause the secretion
of IFN-g in one or other way, and it is enhanced by IL-2, and IL-12 which induce
the NK-cells, and Th cells to form IFN-g. B-lymphocytes need IL-1 to produce
IFN-g. During its secretion, IFN-g influences on the secreting cells as well
as on the cells around through IFN-g-receptors. The first necessary step in
the functioning of the IFN-g pathway is its interaction with receptors located
on the surface of the cells. The receptor for IFN-g is composed of two structurally
homologous polypeptides, called, IFN-g-Ra, and IFN-g-Rb. Signal transduction
is mediated by Jak1, Jak2 and STAT1a, finally transcriptional events are initiated
(Janeway et al., 2005) (see Fig. 2).
Fig. 2 IFN-gamma signaling pathway:
Interferon gamma is secreted from CD4+ Th1 cells, cytotoxic CD8
cells, Th0-cells and activated NK cells. It plays a role in activating
lymphocytes to enhance anti-microbial and anti-tumor effects. In addition
it plays a role in regulating the proliferation, differentiation, and
response of lymphocyte subsets. Signaling takes place through a IFN Recpetor
complex consisting of two alpha chains (Type I receptor) and two beta
chains (Type 2 receptor). Upon phosphorylation by Jak1, Stat1(alpha) transduces
the signal into transcriptional events (Image Permit
Pending http://www.biocarta.com) |
Immunoregulatory functions of IFN-g:
1. IFN-g can stimulate both defending or pathological effects. IFN-g induces the differentiation process of myoloid cells in bone marrow forming cells by up-regulating the high affinity signaling receptor for IgG, called Fc-g-RI. IFN-g also activates the antibody-dependent cytotoxins implemented by the matured granulocytes (Plotnikoff, 1999).
2. IFN-g is the principal macrophage-activating factor (MAF) and provides the means by which T cells activate macrophages to kill phagocytosed microbes. This also increases macrophages’ antitumor activities. Suppression of intracellular parasites under the influence of IFN-g takes place in the nonmacrophage cells as well (Fitzgerald et al., 2001).
3. IFN-g also reinforces the antitumor activities of the cytotoxic lymphocytes. IFN-g together with lymphotoxins-CD4 or CD8, produced by lymphocytes, supress the tumor cell growth. IFN-g induces the expression of the receptors of lymphotoxins by acting in the nucleus of the target cells (Abul et al., 2000).
4. IFN-g increases MHC I expression and, in contrast to Type I interferons, also causes a wide variety of cells to express class MHC II. Thus, IFN-g extends the recognition phase of the immune response by promoting the activation of class II restricted CD4+ helper T cells. In vivo IFN-g can enhance both cellular and humoral immune responses through these actions at the recognition phase (Janeway et al., 2005).
5. IFN-g acts on T lymphocytes to promote their differentiation from naïve CD4+ T cells to Th1 cells. Recall that Th1 cells are involved in the elimination of pathogens residing intracellularly in vesicular compartments (Janeway et al., 2005) .
6. IFN-g is one of the factors which control the differentiation of B-cells. It can either increase or decrease B-cell immune responses. In the late stages for example, IFN-g increases the secretion of immunoglobulin (Janeway et al., 2005).
7. IFN-g plays a very important role in increasing the expression of HLA I and II class molecules on the cell membranes. More over, IFN-g may cause considerable changes on the surface of cell-membrane which inhibits the adhesion and penetration of virus into the cells (Abul et al., 2000).
8. IFN-g stimulates the cytolytic activity of NK cells more so than Type I INF’s (Abul et al., 2000).
9. IFN-g promotes the syntheses of ferment-oligoadenilat synthetases in cells. The polymers of oligoadenilat activates the endogen endonucleases which promotes the destruction of mRNA and rRNA, disturbing the intracellular synthesis in viral cells (Abul et al., 2000).
10. IFN-g promotes the formation of ferment- proteinkinases resulting into the decrease of protein syntheses (Abul et al., 2000).
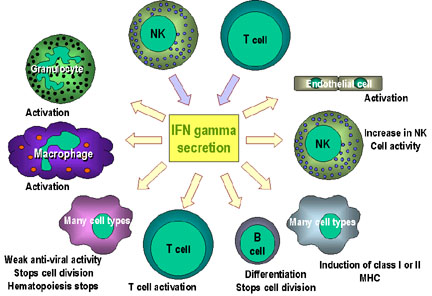 |
Fig. 3 Immunoregulatory actions of interferon gamma on the immune system: - IFN-gamma enhances the microbicidal function of macrophages through
formation of nitric oxide and reactive oxygen intermediates (ROI) -
IFN-gamma stimulates the expression of class I and class II MHC molecules
and co-stimulatory molecules on antigen presenting cells Note: the anti-proliferation and anti-viral activities are weaker than those of IFN alpha and IFN beta. IFN gamma is the most potent of the three at macrophage activation and in inducing class II MHC expression (Image permit pending, http://pathmicro.med.sc.edu) |
The net effect of these varied activities of IFN-g is to promote
inflammatory reactions dependant on macrophages, while inhibiting IgE-dependant
reactions. Knockout mice in which IFN-g or the IFN-g receptor genes have been
disrupted show several immunologic defects, including increased susceptibility
to infections with intracellular microbes. Knockout mice also present reduced
expression levels of MHC II on macrophages after infection with mycobacteria,
reduced levels of IgG2a and IgG3 antibodies, as well as defective NK cell function.
IFN-g medical applications:
Interferons are used therapeutically by injecting them into the blood stream. In 1993, interferon-gamma, received FDA approval for the treatment of a form of multiple sclerosis characterized by the intermittent appearance and disappearance of symptoms (Seppa, 2000). It has also been used to treat chronic granulomatous diseases, an inherited immune disorder in which white blood cells fail to kill bacterial infections, thus causing severe infections in the skin, liver, lungs, and bone. As mentioned in the overview, particular area of interest is the combined use of interferons to enhance other therapies since IFN-g administered simultaneously with interferon alpha increases antibody production (Johnson et al., 2000).
Future studies will focus more on combining interferons with other drug therapies. Research has also shown that these cytokines play numerous roles in regulating many kinds of cell functions. They can promote or hinder the ability of some cells to differentiate, and they can inhibit cell division, which is one reason why they hold promise for stopping cancer growth. Also, biotechnological advances making genetic engineering easier and faster are making protein drugs like interferons more available for study and use. Using recombinant DNA technology, or gene splicing, genes that code for ineterferons are identified, cloned, and used for experimental studies and in making therapeutic quantities of protein. These modern DNA manipulation techniques have made possible the use of cell-signaling molecules like interferons as medicines. Continued research into interferons will continue to expand their medical applications (Johnson, et al., 2000).
References:
Davidson College Biology Department Home Page
© Copyright 2005, Department of Biology, Davidson College,
Davidson, NC 28036
Send comments, questions, and suggestions to: Daniela
V. Alvarez (davillarrealalvarez@davidson.edu).