This page was created as an assignment for my Immunology class at Davidson College.
last updated: 2/18/98

Brief History
It is believed that the malaria parasite has been with us since the dawn of time. Most assume that it originated in AFrica, although there is some speculation that it may have orginated in Asia2-7. In addition, this inveterate relationship is assumed becuase of the persistence of sickly cell disease alleles in the AFrican population, since those infected with SCD also have a reduced level of acute malaria infection8.
The disease appeared as early as 2700BC, when the Chinese cannon of medicine described the classic symptoms of the disease: high fever and enlarged spleen9.
Hippocrates was the first to look beyond the supernatural as the source of malaria9. Pointing to pools of stagnant water (ideal breading grounds for the Anapholes mosquito), he inspired the Romans to begin drainage programs, the first anti-malaria intervention.
It wasn't until the early 1600's, however that treatment was first available to the developed world. Native Peruvian Indians gave Jesuit missinaries bark of the cinchona tree, which contained quinine9. Shortly thereafter, this "Jesuit powder" was avialable in the UK to those suffering from the "auges."
In 1889, Laveran discovered the protozoal cause of malaria, and in 1897, the Anapholes mosquito was demonstrated to be the only known vector of the disease9.
Interestingly, malaria was the most common reason for hospitalization during the Civil War8.
In the 1940's, the discovery of DDT insecticide gave hope for global eradication, as huts and houses world wide were sprayed9. This program saw initial success, as malaria was eliminated or reduced in 77 countries1. However, soon the number of reported cases met and then quickly exceeded previous levels8. There is no singly expanation for the inability to eliminate the disease, but many point to political and social barriers which stunted this eradication program9. Unfortunately, the incomplete nature of these measures only increased selection pressures for insecticide resistant mosquitoes.
Recognizing the almost insurmountable nature of the disease, in 1970, the WHO downgraded the eradication program to a global malaria control program8. The change did little to stop the spread of the disease, as by the mid 70's there was a 2-3 fold increase in cases globally9.
The battle against the Plasmodium parasite and its vector, the Anapholes mosquito has seen little improvement in recent years. As shall be discussed later, drug resistance is now reported in every malaria endemic country; a sobering thought in light of the fact that a widely available vaccine is still not avialable.
Life Cycle of the Plasmodium Parasite9
The four malaria causing species of the Plasmodium parasite (P. falciparum, P. vivax, P. ovale, and P. malariae) have similar life cycles, which can be divided into three stages: I, II, and III.
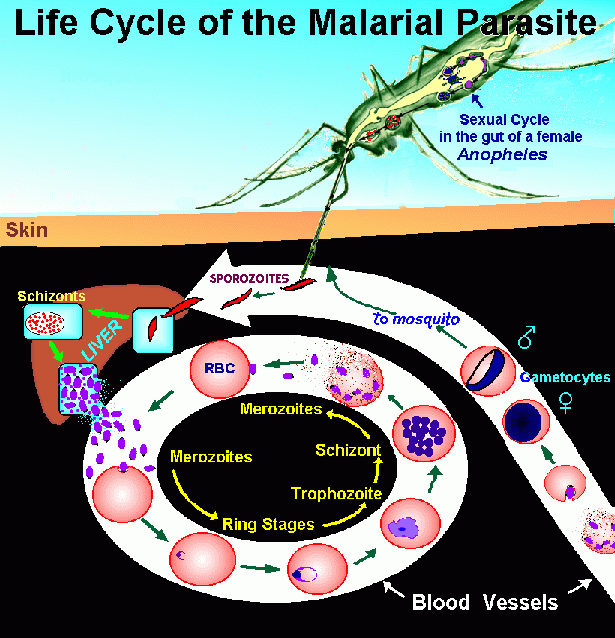
In Stage I, the secretion of anticoagulant into the human host by the female Anapholes mosquito while taking a blood meal also releases the parasitic sporozoites, which are found in the mosquito's salivary gland. Upon entrance to the blood stream, the sporozoites immediately penetrate into the liver cells, where they multiply. After 9-16 days, the life cycle enters Stage II, where the parasitic merozoites rupture from the liver and invade the red blood cells. While inside the RBC's the parasite undergoes erythrocytic schizogony after which the RBC ruptures, releasing either merozoites (which reinvade the liver) or micro- and macrogametocytes (which circulate in the blood stream). In Stage III, the completion of the life cycle, the gametocytes are taken up by a mosquito, where they undergo sexual reproduction. The subsequent fertilization takes place in the mosquito but wall, where an oocyst then develops. Finally, the oocyst ruptures, releasing the sporozoites, which then migrate to the salivary gland, where they await transfer to another host.
Immune Response
Symptoms of malaria can develop anywhere from 10 to 16 days after infection; in some cases, they may never develop10. Typical symptoms include headache, fever, chills, and/or body aches. Also the spleen and liver are often palpable upon clinical examination.
Recent years have seen a signifcant increasse in the still limited understanding of the specifics of the immune respnose to malaria. Both cell mediated and humoral responses are involved in the initial response and natural immunity11, althugh it seems that the immune system never is able to completely kill all the parasites11.
Most important to the immune response seems the production of IgG antibodies. In a 1996 study exploring the role of these cytophilic antibodies and avidity, Ferreira et al. fournd that 1) clinical immune patients displayed a predominance of high-avidity cytophilic antibodies; 2) acutely ill patients showed high levels of cytophilic antibodies as well, but of low avidity; 3) such a response was short lived as antibody levels fell after two months; 4) however, in another study group, affinity maturation was seen in cytophilic antibodies. It has also been shown that IgG antibodies are specific for circumsporozoite proteins on the sporozoites9. This protein is part of a complex pellicle which is responsible for motility and interaction with host plasma membranes12. Once bound to an antigen, IgG1 and IgG2 antibodies bind to Fc RI and Fc RII receptors on monocytes and macrophages13. This binding initiates opsnization and phagocytosis, as well as antibody-dependant cellular inhibition of blood-stage malaria parasites14.
Despite the specificity of the immune system to recognize and kill the Plasmodium parasite, it likewise ahs develped mechanisms intended to counter those of its host. The parasitic gene encoding circumsporozoite protein appears to have high degree of mutation12. This could explain the inability of the immune system to completely rid the body of the parasite. Additionally, upon entrance to the RBC's, the parasite produces adhesion proteins which are expressed upno the cell surface. These proteins cause the blood cells to stick to vessel walls, theoretically to avoid transport to the spleen where the RBC's would be eliminated. Researchers have found that as expected, this region of the parasite chromosome is also highly variable, so that the parasite might escape antibody recognition12.
Drug Therapy
Current drug therapies to malaria infection can be divided into three classes, according to the parasitic stage upon which they act: gametocytocidal drugs (e.g. primaquine) which directly destroy the gametocytes, sporontocidal drugs (e.g. pyrimethamine) which inhibit oocyst develpment in the mosquito, and schizontocides (e.g. chloroquine) which destroy asexual forms of the parasite15.
The mechanisms of the most common antimalarial drugs are mentioned below9:
Primaquine: used primarily against gametocytes and hypnozoites, this supposedly works by inhibiting the parasite's electron transport chain, although the specifics are still not understood.
Pyrimethamin, Proguanil (antifols): These drugs inhibit the action of the enzyme dihydrofolate reductase. This enzyme reduces dihydrofolate to tetrahydrofolate, derivatives of which make one-carbon transfers in many biosynthetic pathways16.
Chloroquine and related derivatives: This classic antimalarial drug attacks the hemoglobin metablism of parasites, possibly by preventing the neutralization f the toxic ferriprotoporphyrin IX. It is proposed that chloroquine is taken up into parasitic food vacuoles and there complees with the mebinder for ferriprotoporphyrin IX, forming a membrane damaging complex, which acts by increasing the parasite's membrane permiability.
Quinine and mefloquine: These drugs cause blebbing of the parasitic membrane.
Sulfonamindes: These drugs inhibit para-aminobenzoic acid (p-ABA) which is needed t synthesize dihydroprerate which is an intermediate compound in the synthesis of tetrahydrofolate. As mentioned above, intermediates of tetrahydrofolate serve as donors of one carbon comounds.
Antibiotics: Many times, antibiotics, such as tetracycline, are used in combination with other therapies when resistant strains of Plasmodium are encountered. It is thought that they affect the parasite's mitochondrial ribosomes, thus disrupting protein sythesis.
Artemisinins: These newest anti-malarials are synthetic versions of traditional Chinese therapies and also seem to affect protein synthesis. They promise to be the next wave of treatment in a never-ending race against drug resistant parasites.
Drug Resistance
Drug resistance has become the major obstacle to malaria control in recent years. The most current definition of drug resistance given by the WHO17 is "the ability of a parasite strain to survivie and/or multiply despite the administratino and absorption of a drug given in the doses equal to or higher than those usually recommended but within the limits of tolerance of the subject." The primary reason for drug resistance has been the "indiscriminate and incomplete" use of drugs accross the world, especially in the poorer endemic regions, leading to heightened selection pressures upon Plasmodium18.
Chloroquine resistance in P. falciparum was first observed in 1961 in Columbia19. Since then, chloroquine reistance has been observed in every part of the world--to such a degree that chloroquine is now considered ineffective against this species unless coupled with antibiotics20. Additionally, resistance to every drug therapy except the new artemisinins has been observed18. Even more unsettling, it seems that resistance has spread beyond the falciparum species and is now observed in each of the other species21.
There is no way to overcome drug resistance, thus for every resistant strain, a new drug therapy must be developed. The most advisable method of treatment in a given region is to use a drug as long as it is viable. Once it loses efficacy, quickly remove it as a treatment, and replace it with a new therapy. Also, it is imperative that drug treatments be taken in completion.
Hopefully once treatments are administered in a more responsible manner, drug resistance will be brought under control, and finally malaria control can become a reality.
For more about malaria, visit these sites:
![]()
Please send questions or comments to me at grnoland@davison.edu.
BACK to GSN's homepage
Link to Davidson College's Biology homepage.