
Feeding behavior comparison of natricine snakes and terrestrial snakes: which senses, chemical \ olfaction or visual, play a larger role in prey detection and selection
Roe, John H. and Temple, John
Biology Dept., Davidson College, Davidson, NC 28036
ABSTRACT:
Because snakes inhabit both terrestrial and aquatic environments, specialization in prey detection strategies that best fit these differential environments should be seen. Snakes have two primary sensing capabilities, vision and olfaction / chemical sensing via the vomeronasal organ. We hypothesized that terrestrial specialists, such as Elaphe obsoleta, would rely on the vomeronasal organ when orienting to and capturing prey, while the aquatic specialists, such as Nerodia sipedon, would rely more on vision. We found that, although it is hard to compare one species behavior to the other, aquatic species showed an intense investigative behavior and struck at chemical stimuli, while the terrestrial species showed no significant reactions to chemical stimuli. These results, along with an observation of the slow reactions of E. obsoleta to a live pinky mouse, allow us to suggest that E.obsoleta might take a slower investigative approach towards their prey, while N. sipedon adopts a more aggressive, accelerated approach. In a visual test, N. sipedon caught more fish and engaged in active, visual searches when hunting a non-cryptically colored prey. When hunting a cryptically colored prey, N. sipedon tongue flicked more and utilized a wait and pounce strategy instead of active visual searching. These observations lead to the conclusion that as the environmental situation changes, the feeding behaviors might change as well.
INTRODUCTION:
Species of the suborder Serpentes have evolved sensitive perceptual mechanisms to help in conspecific recognition, locomotion, reproduction, homing in on habitat, antipredator behavior and prey selection and handling (Burghardt, Ford 1993). Because the snakes have radiated into a diversity of habitats, they have subsequently adapted to the conditions in which they live. Therefore, it would appear that some snakes would have developed particular sensory capabilities that are more advantageous for prey selection and detection according to the environmental conditions in which they evolved. It has been suggested that two sensory mechanisms play the major role in feeding behavior of snakes: the visual and the chemical \ olfaction. The importance of such sensory mechanisms in a variety of snakes has been studied. Drummond found that in ingestively naÔve garter snakes, genus Thamnophis, vision is not used in the elicitation of attack, but stimulates investigative behavior such as tongue flicking and orientation. He found that chemical cues alone are sufficient to elicit an attack (Drummond 1985). However, Drummond also found that piscivorous taxa, such as Nerodia sipedon and water specialists in the genus Thamnophis, attacked fish models where there was no odor present. Although it has been found that both chemical and visual sensing, when used together, result in the most efficient feeding behavior, it is also apparent that one sensory mechanism might be of more importance than the other across a variety of snakes occupying different habitats. By observing differential feeding behaviors among terrestrial specialists and aquatic specialists, we expect to see different roles of vision and chemical \ olfaction in prey selection and detection. The terrestrial species are expected to rely on chemical \ olfaction, while the aquatic specialists are expected to rely more on vision.
METHODOLOGY :
Snakes for comparison
Natricine - Nerodia sipedon, Northern Water Snake - Forages mostly on fish, frogs and toads, tadpoles, salamanders, snakes and lizards, but will also take a variety of terrestrials including earthworms, leeches, slugs, snails, insects, millipedes, spiders and crayfish. Found in any freshwater body within its range. Activity mostly nocturnal, but will readily eat during daylight. (Figure 1)
Terrestrial - Elaphe obsoleta quadrivittata, Yellow Rat Snake - Forages on shrews, rats, mice, squirrels, weasels, birds, lizards, snakes, snails, beetles, caterpillars and frogs. Diurnal activity. Inhabits a variety of wooldand and scrub habitats including deciduous forests, scrub pine woods and swamp and marsh borders. (Figure 1)

Figure 1. Above are the snakes of comparison. Above is Elaphe obsoleta; below is Nerodia sipedon. Both snakes are seen here at about eight weeks old.
Housing
All snakes were received as newborn, the N. sipedon from NC State, the E. obsoleta from Glades Herps. Snakes were kept in the Animal Facilities on the third floor of Dana Science building at a temperature of 22oC, and 50% humidity. A twelve hour light / dark cycle was also maintained. Snakes were housed individualy in plastic containers measuring 10*5*4 inches, containing a paper towel and a water dish. E. obsoleta were fed one pinky mouse once a week, while the N. sipedon were fed two Rosy Red Feeder fish each week. Snakes were deprived of food for one week prior to testing. All testing was conducted in room 305, a room just across the hall from the animal housing facility, between 1400 and 1800 hrs. This room varied from 22.5oC to 25.7oC on test days. Thirty minutes prior to testing, the snakes were moved into the testing room, where they could habituate to the change in environment. Chemical / olfaction testing was conducted when the snakes were five weeks old, while the visual tests were conducted when the snakes were seven weeks old.
Methods
Chemical / Olfaction Test
To test the role of chemical \ olfaction sensing, methods utilizing prey extract described by Brughardt were employed (1968). Prey extracts were prepared from newborn pinky mice and Rosy Red feeder fish. These specimens were first placed in a freezer for fifteen minutes, then used in the preparation of chemical extracts. Specimens were first washed in tap water, blotted dry, then weighed to the nearest .01 gm. Distilled water in the proportion of 10ml per 1.5 gm of prey specimen was brought to 52 0C before the animal was placed in the water and stirred for one minute. The resulting solution was then filtered so as to remove any cell particles, hair or other animal parts from the solution. The resulting liquid had the appearance of water, and smells could not be distinguished by the human nose. Extracts were then placed in the refrigerator until test time.
Snakes were tested in the same plastic containers in which they were kept. A cardboard partition was constructed around the containers on three sides to block outside distractions, while the third side was left open for the presentation of the stimuli. Prey extracts, as well as control solutions consisting of distilled water, were brought to room temperature, and presented on six inch cotton swabs. Swabs were first dipped in the extract or control vile, given a quick shake, then slowly moved to within two centimeters of the snake's snout. For sixty seconds, tongue flicks were counted, and if a strike occurred, the amount of time elapsed between the stimulus presentation and the strike was recorded. If the snake failed to tongue flick or strike within thirty seconds, the swab was brought closer to touch the snake's snout three times, then moved back to the two centimeter distance. Each of the ten snakes was tested in both the control and extract treatments over a three day period, where the order of the treatments was selected randomly. This procedure was repeated one week later in order to obtain a second set of data for each snake.
Each snake is scored based on a formula derived by Burghardt (1968). Tongue flicking and attacks are included in the score, which is derived by the formula:
Score = base unit + (60- response latency)
The base unit is the maximum number of tongue flicks by an individual that did not strike the swab in a trial. The response latency term accounts for the amount of time a snake takes to attack, therefore acting as an indicator of the interest in feeding. Presumably, the smaller the response latency, the more interested the snake is in feeding. Since attacks are considered to be a more definitive feeding behavior than tongue flicks, no snake that did not attack could score higher than one that did attack. For snakes that did not attack, their score was simply be the amount of tongue flicks observed.
Cryptic / Non-Cryptic Test
To test the role of vision, a method similar to that used by Teather (1991) was employed. Natricine snakes of the genus Nerodia were observed while feeding on Rosy Red feeder fish in two test aquariums. The aquariums backgrounds were manipulated so as to differ in the extent that prey were visible. Four sides of the aquariums were covered with white paper to limit outside disturbances, while the top and one side were left open for viewing behavior. At the end opposite the observer, a separate background color was fixed to the aquarium. One aquarium received a blue background, where the fish would stand out in contrast to the surroundings, while the other aquarium contained a mottled camouflage background pattern, where the fish would be cryptically colored to blend in with the surroundings (Figure 2).
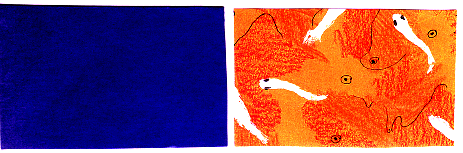
Figure 2. The feeding backgrounds were designed to vary to the extent to which prey (rosy red feeder fish) were visible. The blue background stood in contrast to the fish, while the orange background allowed the fish to blend in with their surroundings.
The observer sat behind a 40*40 inch cardboard barrier with a 2*5 inch viewing slot. Both aquariums were tilted by placing a brick under the end closest to the observer, allowing the water, when introduced, to fill the ends of differing backgrounds, while leaving the end closest to the observer dry. At the beginning of every trial, 500ml of tap water, along with ten fish, was placed in the aquarium. One snake was introduced to the edge of the water, and feeding behavior was observed and recorded. The amount of time spent in the water and the time spent foraging were recorded. Any behavior that involved the snake's head being underwater was considered foraging behavior. Also, the number of tongue flicks, as well as the number of fish captured were recorded. And finally, the time elapsed before the first capture was measured. Each snake was tested in both aquariums over a three day period. The order in which the snakes were tested was selected randomly so as to limit the effect that learned behavior, or a full stomach, might have on the second trial. This procedure was repeated one week later in order to obtain two sets of data for each snake.
Data Analysis A Wilcoxon / Kruscal-Wallis Rank Sign Test, as well as a simple T test, was used to analyze data. JMP Statistical analysis software was used to run tests.
RESULTS:
Chemical / Olfaction Test
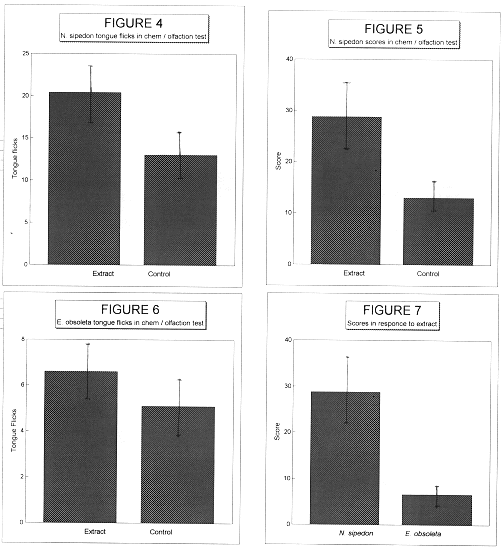
When the N. sipedon control values are compared with the extract values for both score and tongue flicks, an obvious trend becomes evident. N. sipedon tongue flicked significantly more times when presented with a prey extract stimulus than when presented a control swab (P value = .012, Figure 4). Likewise, the scores were significantly higher for extract stimulus over those of the control (P value = .002, Figure 5). Out of seventeen trials with prey extract stimulus, five resulted in a strike, while no strikes were observed in the control treatments. In E. obsoleta, there was no difference in the tongue flicks or score values between the control and extract trials (P value = .134, Figure 6). Additionally, out of twenty trials, no strikes were observed in either treatment. In comparing across species, similar results are seen. N. sipedon tongue flicked significantly more and recorded significantly higher scores than E. obsoleta (P values = .017, .011, Figure 7) It is interesting to note that N. sipedon tongue flicked more frequently than E. obsoleta in the control treatments.
Cryptic / Non-Cryptic Test
The N. sipedon feeding against the non-cryptic background spent significantly more time in the water (P value =.014, Figure 8) and significantly more time foraging (P value =.042, Figure 9) than did those snakes feeding against the cryptic background. Snakes feeding in the cryptic treatment tongue flicked significantly more than those in the non-cryptic treatment (P value =.0002, Figure 10). Snakes feeding in the cryptic background were less successful in capturing fish than those in the non-cryptic treatment, but the value was not statistically significant (P value =.07, Figure 11). This value, however, is approaching significance, so a possible trend in capture success is evident. There was no difference in the amount of time elapsed before the first capture (P value = .1072, Figure 12), nor was there a difference in the capture success rate (P value =.116, Figure 13) between the two experimental feeding conditions.


DISCUSSION:
When comparing the behaviors of the natricine N. sipedon to those of the terrestrial E. obsoleta in the chemical / olfaction trials, N. sipedon seemed more highly motivated to investigate and strike at a swab with prey extract. This conclusion is supported by the higher tongue flick responses and scores, as well as the amount of strikes by the aquatic specialist. This result contradicts our initial assumption that the terrestrial species would rely more on olfaction and chemical sensing in locating prey, and therefore show higher scores and tongue flicks. Although our results allow us to posit that chemical sensing might play a more important role in an aquatic specialist's than previously assumed, it cannot be concluded that N. sipedon relies more on chemical sensing by the vomeronasal organ that E. obsoleta in detecting and manipulating prey. There appear to be at least two reasons which might explain the results. First, because each species was presented different prey extracts, we cannot assume that these extracts were of the same potency. Although both extracts were made in the same proportions, the relative odor and chemicals dissolved into solution could have varied significantly. Both prey were of equal weight, but the amount of cellular matter, mucus or excretions which were stirred into the water may have been different. If one of the extracts were more potent, the snake species to which this was presented would have more incentive to investigate and strike. For future experiments, either a method of diluting extracts to equal potency, or the presentation of a single prey extract to the two snake species, should be applied. A second reason which might explain the results was observed in the differential investigation of possible prey by N. sipedon and E. obsoleta. The rat snakes seemed to use a long, investigative approach when fed pinkies, whereas the water snakes did not. It was a rare occurrence when a rat snake began ingesting its prey within the first fifteen minutes after a live pinky mouse was introduced to the container, but most Rosy Red feeder fish were eaten within one minute of being introduced to the feeding dish of N. sipedon. Rat snakes would sometimes investigate the mouse very closely, leave it alone, and neglect to eat it throughout the three days the mouse was alive. N. sipedon, on the other hand, never failed to eat all fish offered. If rat snakes showed such a slow reaction to a live prey item, where both visual and chemical clues were present, it seems logical that the investigative approach to the cotton swab would be even longer. Therefore, the time allotment of sixty seconds per trial would probably not be long enough to elicit intense tongue flicking or strikes. To compare these score and tongue flick values across species is a precarious endeavor, for the relative potencies of extracts, as well as the behavioral differences that exist, make the two values difficult to relate. Values which can be compared with confidence, however, are the tongue flicks and scores of snakes in the control treatments. Here, we see that N. sipedon tongue flicked more than E. obsoleta , providing evidence that N. sipedon might display faster investigative behavior to inanimate objects with no scent association.
When examining the feeding behavior of N. sipedon as it hunts against backgrounds which vary to the extent which prey are visible, a trend becomes evident. As the fish become more visible, the snakes engage in visual oriented searching methods, and when fish become less visible, olfactory searching techniques are adopted. Because snakes spent significantly more time in the water, as well as more time foraging in the non cryptic trials, we concluded that snakes might be able to see the non-cryptically colored fish more easily, and therefore engage in more visually oriented prey detection behaviors. If prey stood out in contrast to the background, active visual searches would be expected. It would also be expected that snakes would have increased success as the prey become more visible, and in fact, snakes in the non-cryptic trials caught an average of 2.3 fish, where snakes in the cryptic trials averaged 1.4 captures. Although this difference in capture success was not significant, it approaches significance (P value = .07). When the prey were less visible in the cryptic trials, olfactory oriented prey detection behaviors were used, for snakes tongue flicked significantly more when feeding against a cryptic background. With prey not visible, snakes might find it advantageous to tongue flick in order to orient to prey. Rather than expend energy by actively searching for prey when prey is not visible, a better strategy would be to tongue flick in order to orient to its prey, then remain still and wait for prey to enter a zone where snakes could then use vision to strike. This strategy was witnessed many times in the cryptic trials. This would account for the small amount of time spent foraging, for snakes only dipped their head in the water for a split second while striking. This tactic also accounts for the small amount of time spent in water, for snakes awaited prey while positioning themselves on the edge of the water, only entering the water to strike at prey. In the water snake, we see evidence that, as the feeding environment changes, the relative importance of sensory mechanisms used in prey detection and capture might change as well. Although it has been proposed by Drummond (1983) that "superior visual accommodation is a key morphological adaptation associated with aquatic specialization", we cannot confidently conclude from our data that N. sipedon relies more on vision than olfaction in feeding behavior. Our data do allow us to conclude that, in certain situations, N. sipedon relies on vision, while in other situations, olfaction may be of more importance. To further test the differential feeding behaviors of terrestrial and aquatic snakes, an experiment needs to be designed to examine the role of vision in terrestrial snakes. A similar method to the cryptic non-cryptic trials used to test N. sipedon was tried, but the rat snakes' slow reaction to an introduced stimulus made it hard to observe feeding behavior. As in all experiments conducted in a laboratory setting in the presence of human observers, one can never be certain that behaviors are representative of wild populations. To test these snakes in a natural setting, or as near to natural as possible, would be extremely difficult, but could provide observations of behaviors not seen in the laboratory setting. One complicating factor in the chemical / olfaction test was the smell of the testing room. The room is used for storage of invertebrate specimens, so strong preserving fluids can be smelled. This smell could have had an effect on the snakes detection of extract scent, thus affecting the snakes reaction. In the visual test, because snakes were tested over a three day period, the number of fish the snake captured and ate in an early trial could have affected its motivation to eat in the second trial. Although all snakes were starved for one week prior to testing, and although the water snakes seemed ready to eat at all times, this experimental hitch could have also affected our results. Finally, because our experiment only examined the feeding behavior of young snakes (less than two months old), our results might not reflect a true representation of snake populations. As a snake matures, a shift in the sensing mechanisms important for locating prey might occur. With increasing experience and learned behaviors, methods used to detect and capture prey might cause a change in feeding behavior as well. To further test differences in feeding behaviors in aquatic and terrestrial species, multiple age groups should be examined.
ACKNOWLEDGMENTS:
I would like to thank Amy Becton for her help in the animal housing facilities, Dr. Peroni for her help in statistical analysis, and Dr. John Temple for his expertise in herpetology. Dr. Gordon Burghardt also contributed information on experimental design.
LITERATURE CITED:
Barbour, R.W. Earnst, C.H. Snakes of Eastern North America. George Mason Univ. Press, Fairfax, Va: 1989.
Burghardt, G.M. Comparative Prey - Attack Studies In Newborn Snakes Of The Genus Thamnophis. Behavior, 1968, 33:77-114.
Burghardt, G.M. Ford, N.B. Perceptual Mechanisms and the Behavioral Ecology of Snakes. Snakes: Ecology and Behavior , Seigel and Collins eds. McGraw-Hill, Inc. NY, NY: 1993. Pp117-164.
Drummond, H. Aquatic Foraging in Garter Snakes: A Comparison of Specialists and Generalists. Behavior, 86: 2-30. Drummond, H. The role of vision in the predatory behaviour of natricine snakes. Animal Behavior, 1985, 33:206-215.
Teather, K.L. The Relative Importance of Visual and Chemical Cues for Foraging in Newborn Blue-Striped Garter Snakes (Thamnophis sirtalis sirtalis). Behavior, 1991, 117 (3-4):255-261.
WORKS CONSULTED:
Burghardt, G.M. Responses to Chemical Stimuli of Prey in Newly Hatched Snakes of the Genus Elaphe. Animal Behavior, 1971, 19: 486-489.
Henderson, R.W. Binder, M.H. and Burghardt, G.M. Responses of Neonate Hispaniolan Vine Snakes (Uromacer freantus) to Prey Extracts. Herpetologica, 1983, 39:75-77.
Herzog, H.A. and Burghardt, G.M. Prey movement and Predatory Behavior of Juvenile Western Yellow Bellied Racers, Coluber constrictor mormon. Herpetologica, 1974, 30: 285-288.
Porter, R. H. and Czaplicki, J.A. Responses of Water Snakes (Natrix r. rhombifera) and Garter Snakes (Thamnophis sirtalis) to Chemical Cues. Animal Behavior, 1974, 2: 129-132.