IMMUNOFLUORESCENCE
Molecular Localization in Cells and Tissues
The characteristic stability of antibody binding to specific
antigen lends itself as an immunological probe for localization of particular
molecules in cells and tissues. Because fluorescent dyes such as fluorescein
 and
rhodamine can be coupled to antibodies without destroying their
specificity, the conjugates can complex with antigen and be visualized
via fluorescence
microscopy. The microscope excites the chosen dyes by light of one
or more wavelengths, which in turn emits light at a characteristic wavelength
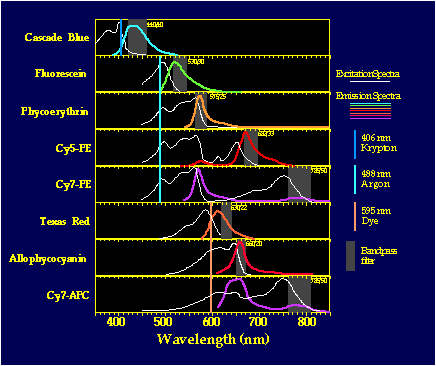
captured by a selective filter. Figure 1 shows the excitation and emission
spectra for 8 different dyes used in FACS
immunofluorescence experiments. The image is then projected with localized
regions of fluorescence indicating different antigens labeled by antibodies
of distinctive color.
and
rhodamine can be coupled to antibodies without destroying their
specificity, the conjugates can complex with antigen and be visualized
via fluorescence
microscopy. The microscope excites the chosen dyes by light of one
or more wavelengths, which in turn emits light at a characteristic wavelength
captured by a selective filter. Figure 1 shows the excitation and emission
spectra for 8 different dyes used in FACS
immunofluorescence experiments. The image is then projected with localized
regions of fluorescence indicating different antigens labeled by antibodies
of distinctive color.
The test can be carried out in three general ways (Figure
2): (1) Direct test, (2) Indirect test, and (3) Sandwich test.
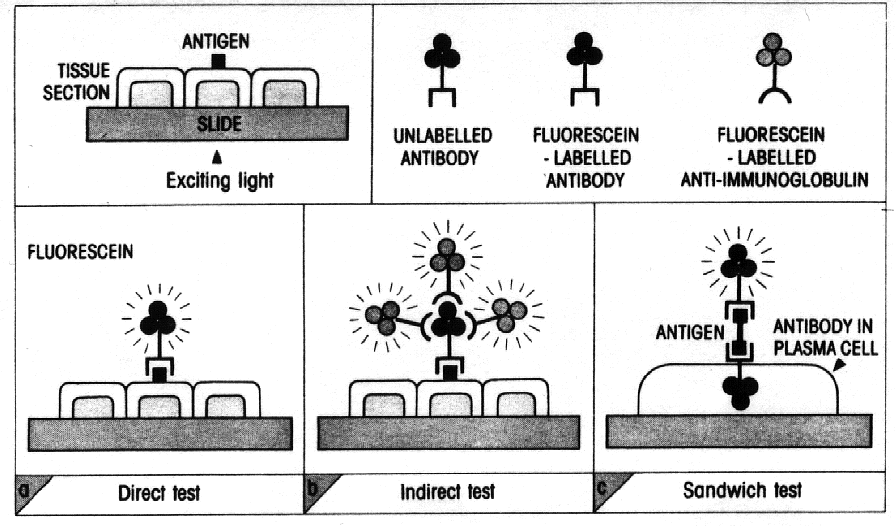
Figure 2 : from Essential Immunology, Ivan
M. Roitt.
The Direct Test
The antibody is itself conjugated with the fluorochrome
and applied directly to a monolayer of cells or to frozen tissue on a slide.
When examined with a fluorescence microscope, the antibody labelled with
the fluorescent moiety identifies the localized antigen. Two different
antigens can be identified simultaneously, in the same preparation by using
antisera conjugated to dyes active at different wavelengths. Direct immunofluoresence
imaging in Figure 3 indicates deposition of material containing polyclonal
IgG and complement within capillary walls and mesangium, as well as tubular
basement membranes.
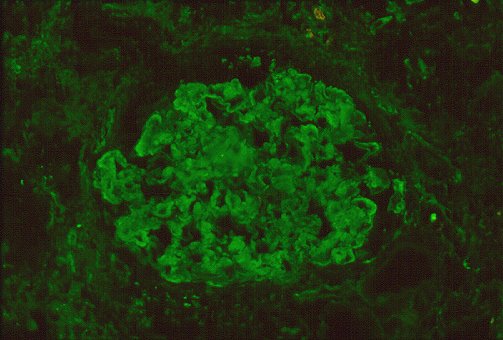
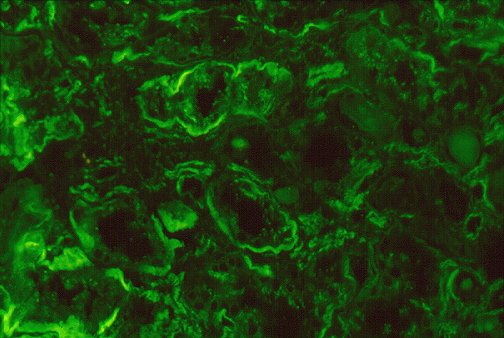
Figure 3 : photographs by Maria Parizhskaya, M.D. and Sheldon
Bastacky, M.D.
 Click
here for a link to a direct immunofluorescence staining protocol, including
methods, materials, and equipment needed.
Click
here for a link to a direct immunofluorescence staining protocol, including
methods, materials, and equipment needed.
The Indirect Test
Unlike the direct test, the indirect test is a double-layer
technique. The unlabelled antibody is applied directly to the tissue substrate
and then treated with a fluorochrome-conjugated anti-immunoglobulin serum
(figure 4). There are several advantages to this technique, and is recently
much favoured over the direct test.
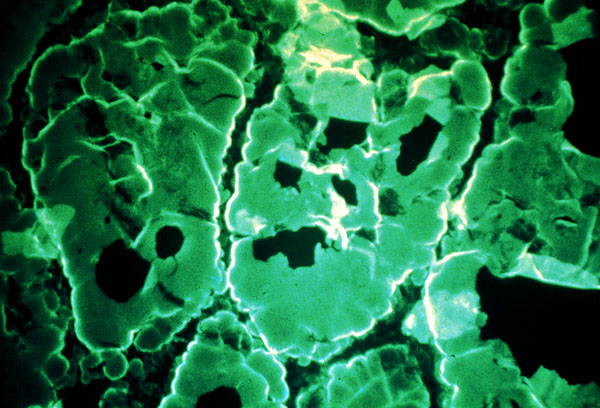
Figure 4 : Goat antisera, tagged with fluorscein, made against human
IgG, was used to detect human autoantibody in thyroid tissue. The test
in this case is positive for anti-thryroglobulin in the thyroid follicle
colloid. (click
here for link to source)
Because several fluorescent anti-immunoglobulins can bind
to each antibody present in the first layer, the fluorescence is brighter
than the direct test. The method is also more flexible because a semi-quantitative
assessment of the distribution of classes and subclasses of antibodies
can be made by using antisera conjugates to individual immunoglobulin heavy
chains. It is also more time-efficient since it is only one signal labelled
reagent, the anti-immunoglobulin, is prepared during the lengthy conjugation
process. The test is also not limited to localization of antibodies. Complement
fixation can also be assessed by adding a mixture of the first antibody
plus a source of complement, followed by a fluorescent anti-commplement
reagent as the second layer. Application of a third layer increases the
sensitivity of the test (unfortunately at the cost of specificity).
 Click
here to connect to an indirect immunofluorescence staining protocol, including
methods, materials, and equipment needed.
Click
here to connect to an indirect immunofluorescence staining protocol, including
methods, materials, and equipment needed.
The Sandwich Test
This test was designed to visualize a specific antibody
produced within a cell. The tissue is first fixed with ethanol to prevent
washing away the antibody during the test. Treatment with polysaccharide
antigen constitutes the first layer. After washing, the fluorochrome-labelled
antibody to the antigen is added to locate cells which have specifically
bound the antigen (see Figure 2).
Cellular and molecular immunology. Abul K. Abbas, Andrew H. Lichtman,
Jordan S. Pober. Philadelphia : W.B. Saunders, c1994 (60-61).
Essential immunology. Ivan M. Roitt. Oxford; Boston : Blackwell
Scientific Publications; St. Louis, MO.: Distributors, USA, Mosby-Year
Book, 1991 (96-100).
Immunobiology. Charles A. Janeway, Jr., Paul Travers. New York
: Current Biology Ltd., 1997 (2:22-2:23).
Kansas University Medical College :
Stanford University :
University of Florida, Flow Cytometry Core Lab :
University of Pittsburgh Medical College :
 Return
to Immunology Home Page
Return
to Immunology Home Page
 Return
to My Home Page
Return
to My Home Page
 Direct
comments and suggestions to Tehnaz Parakh.
Direct
comments and suggestions to Tehnaz Parakh.
 and
rhodamine can be coupled to antibodies without destroying their
specificity, the conjugates can complex with antigen and be visualized
via fluorescence
microscopy. The microscope excites the chosen dyes by light of one
or more wavelengths, which in turn emits light at a characteristic wavelength
captured by a selective filter. Figure 1 shows the excitation and emission
spectra for 8 different dyes used in FACS
immunofluorescence experiments. The image is then projected with localized
regions of fluorescence indicating different antigens labeled by antibodies
of distinctive color.
and
rhodamine can be coupled to antibodies without destroying their
specificity, the conjugates can complex with antigen and be visualized
via fluorescence
microscopy. The microscope excites the chosen dyes by light of one
or more wavelengths, which in turn emits light at a characteristic wavelength
captured by a selective filter. Figure 1 shows the excitation and emission
spectra for 8 different dyes used in FACS
immunofluorescence experiments. The image is then projected with localized
regions of fluorescence indicating different antigens labeled by antibodies
of distinctive color.



