This web page was created as an assignment for an undergraduate course at Davidson College.
My Favorite Protein
Adenylyl Cyclase
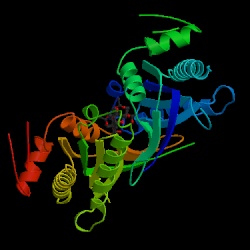
Fig.1. This is a three-dimensional chime image of adenylyl cyclase. Image courtesy of Protein Data Bank, permission pending.
Communication between cells is essential to life. Messages are continuously being sent throughout your body in response to some stimulus. As you read this text a message is being sent from your eyes via nerve cells to your brain, where it is interpreted. Adenylyl cyclase plays a very important role in such cellular communication pathways. This enzyme catalyzes the conversion of ATP to cyclic AMP (cAMP). Adenylyl cyclase is an integral membrane protein. Structural analysis has shown that it is made up of two groups of six transmembrane units, each with a catalytic domain on the cytosylic side(Cooper, et. al, 1995). The adenylyl cyclase signal system includes three plasma membrane-bound proteins: a G protein-receptor, a trimeric G protein (stimulatory or inhibitory), and adenylyl cyclase (Taussig, et. al, 1995). The G protein-receptor specificity may be for a hormone, neurotransmitter, or some other chemical signal, and thus it controls the activity of the system. When a ligand binds, the receptor becomes activated and binds with the trimeric G protein. This causes the G protein to pick up a GTP. Then, the activated portion of the G protein separates from the rest of the protein and diffuses along the cytosol side of the plasma membrane until it encounters adenylyl cyclase. This enzyme then begins converting cAMP from ATP. Cyclic AMP is a small molecule that acts as a second messenger within cells to carry a stimulus from an extracellular receptor to an intercellular target. It amplifies the signal because for every ligand that binds, many cAMP molecules are synthesized.
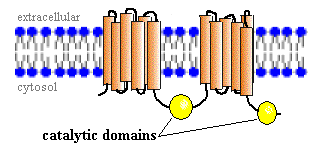
Fig. 2. A schematic drawing of adenylyl cyclase, showing the two transmembrane units and the two catalytic domains on the cytosol side of the plasma membrane. Image courtesy of http://www.vivo.colostate.edu/hbooks/molecules/cyclase.html, permission pending.
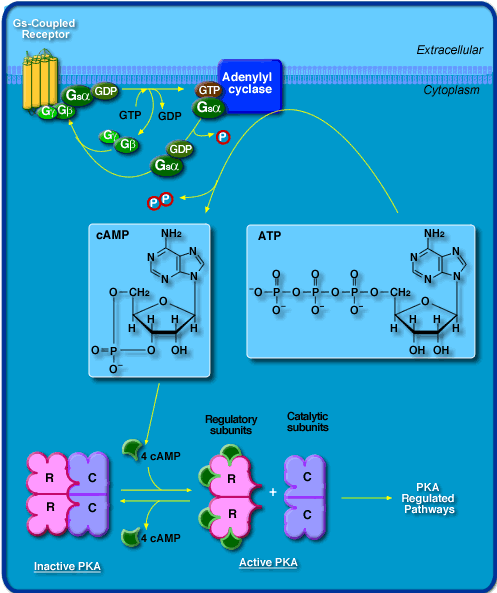
Fig. 3. A schematic drawing of the adenylyl cyclase system. In yellow is the G protein-receptor, green is the trimeric G protein, and blue is adenylyl cyclase. The bottom of the drawing shows how cAMP interacts with intracellular protein kinases. Image courtesy of http://beagle.colorado.edu/courses/3280/chime/rsmin/right-a.htm, permission pending.
Adenylyl cyclase is located in many different tissues throughout the body. Nine different isoforms have been identified, and they are expressed in different proportions in the various tissues. About 50% of the overall amino acid sequence is conserved in the different isoforms. The amino acid sequence of the catalytic domain is conserved up to 93%. This section is also highly conserved in Drosophila and Dictyostelium adenylyl cyclases (Taussig, et. al, 1995).
Regulation of adenylyl cyclase is mediated by several different means. All isoforms are activated by the GTP-bound a subunit of the stimulatory G protein, as stated above. They are also variously regulated by Ca2+, forskolin, protein kinase C (PKC), and ßy subunits of G proteins (Bowen, 2000). The following is a breakdown of the different isoforms of adenylyl cyclase:
Type I
Expressed heavily in the hippocampus and granular layer of the cerebellum
Stimulated by Ca2+/calmodulin
Inhibited by ßy G proteins
Mildly stimulated by a G proteins (Cooper, et. al, 1995)
Located on chromosome 7, mouse homolog on chromosome 11 (OMIM,
1998)
Type II
Expressed in cerebellum
Stimulated by ßy G proteins and a G proteins and PKC
Not activated by Ca2+ (Cooper, 1995)
Located on chromosome 5, mouse homolog on chromosome 13 (OMIM,
2001)
Type III
Expressed predominantly in olfactory neuroepithelium, lungs; mildly expressed
everywhere
Stimulated by Ca2+/calmodulin and a G proteins
Located on chromosome 2, mouse homolog on chromosome 12 (OMIM,
2002)
Type IV
A rare form of adenylyl cyclase
Stimulated by ßy G proteins and a G proteins
Not activated by Ca2+ (Cooper, 1995)
Located on chromosome 14 (OMIM,
2001)
Type V
Expressed in the caudate nucleus
Stimulated by a G proteins
Inhibited by Ca2+ (Cooper, 1995)
Located on chromosome 3 (OMIM,
2001)
Type VI
Expressed in heart
Stimulated by a G proteins
Inhibited by Ca2+ (Cooper, 1995)
Located on chromosome 12 (OMIM,
2001)
Type VII
Expressed in granular layer of cerebellum
Stimulated by a G proteins and PKC
Not activated by Ca2+ (Cooper, 1995)
Type VIII
Expressed in hippocampus
Stimulated by Ca2+
Mildly stimulated by a G proteins (Cooper, 1995)
Located on chromosome 8 (OMIM,
1999)
Type IX
A rare form of adenylyl cyclase
Evolutionarily, the different forms of adenylyl cyclase allow many different
signals to be transferred using the same mechanism. The overall structure
of the protein is maintained between species; however, receptor specificity
adapts it for different functions in different cells. The complexity of this
molecule is still being discovered, for more information on my favorite protein,
adenylyl cyclase visit NCBI.
References:
Bowen, R. Biomedical Hypertexts. Adenylyl Cyclase. Aug 3, 2000. Mar 12, 2003. <http://www.vivo.colostate.edu/hbooks/molecules/cyclase.html>
Cooper, D.M.F., Mons, N., and J.W. Karpen. (1995). Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature. 374, 421-424.
Online Mendelian Inheritance in Man, OMIM ™. Johns Hopkins University, Baltimore, MD. MIM Number:{103072}:{Jun 24, 1998}:. <http://www.ncbi.nlm.nih.gov/omim/>
Online Mendelian Inheritance in Man, OMIM ™. Johns Hopkins University, Baltimore, MD. MIM Number:{103070}:{Jul 9, 1999}:. <http://www.ncbi.nlm.nih.gov/omim/>
Online Mendelian Inheritance in Man, OMIM ™. Johns Hopkins University, Baltimore, MD. MIM Number:{103071}:{May 17, 2001}:. <http://www.ncbi.nlm.nih.gov/omim/>
Online Mendelian Inheritance in Man, OMIM ™. Johns Hopkins University, Baltimore, MD. MIM Number:{600291}:{Apr 10, 2002}:. <http://www.ncbi.nlm.nih.gov/omim/>
Taussig, R. and A.G. Gilman. (1995). Mammalian membrane-bound adenylyl cyclases. Journal of Biological Chemistry. 270, 1-4.
email questions and comments to: tamaloney@davidson.edu