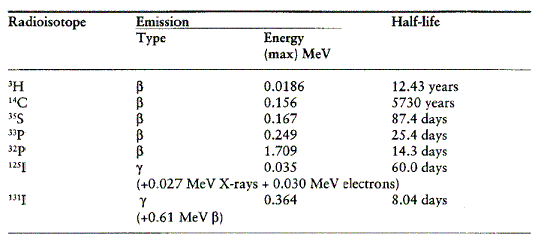
Radioisotopes are atoms with unstable nuclei that decay over time, emitting detectable radiation. In biology, radioisotopes are often incorporated into molecules of interest so that those molecules can be followed by following their radiation trail. The table below lists radioisotopes used often in biology with their corresponding half-lives and the energies of their emitted radiation (Laskey, 2003).
Table 1. Half-lives and radiation energies of radioisotopes used often in biology ( Laskey, 2003). Click on the table to view the document from which it was obtained in Adobe Acrobat PDF format. Permission for use of this table is pending.