GnT-V
Glycobiology Background:
Glycobiology, or “the biology of sugars,” is becoming an increasingly vital field in the quest to understand cellular communication, especially looking at cell-to-cell interactions. Sugars on the outside of cells are involved in a vast range of cell functions, and perhaps most intriguing are the interactions that occur during oncogenesis, or the initiation and spread of cancer. The association between glycosylation and oncogenesis followed by metastasis has been recognized for at least ten years (Couldrey, et al. 2000). Specifically, the enzyme N-acetylglucosaminyltransferase V (GnT-V) has been found to be involved with cancers, and is therefore an important protein to examine. In fact, understanding this enzyme’s role in the spread of cancer may lead to the discovery of an effective method of keeping cancers localized in a patient, and thus making the cancer easier to eradicate through chemotherapy.
GnT-V Specifics:
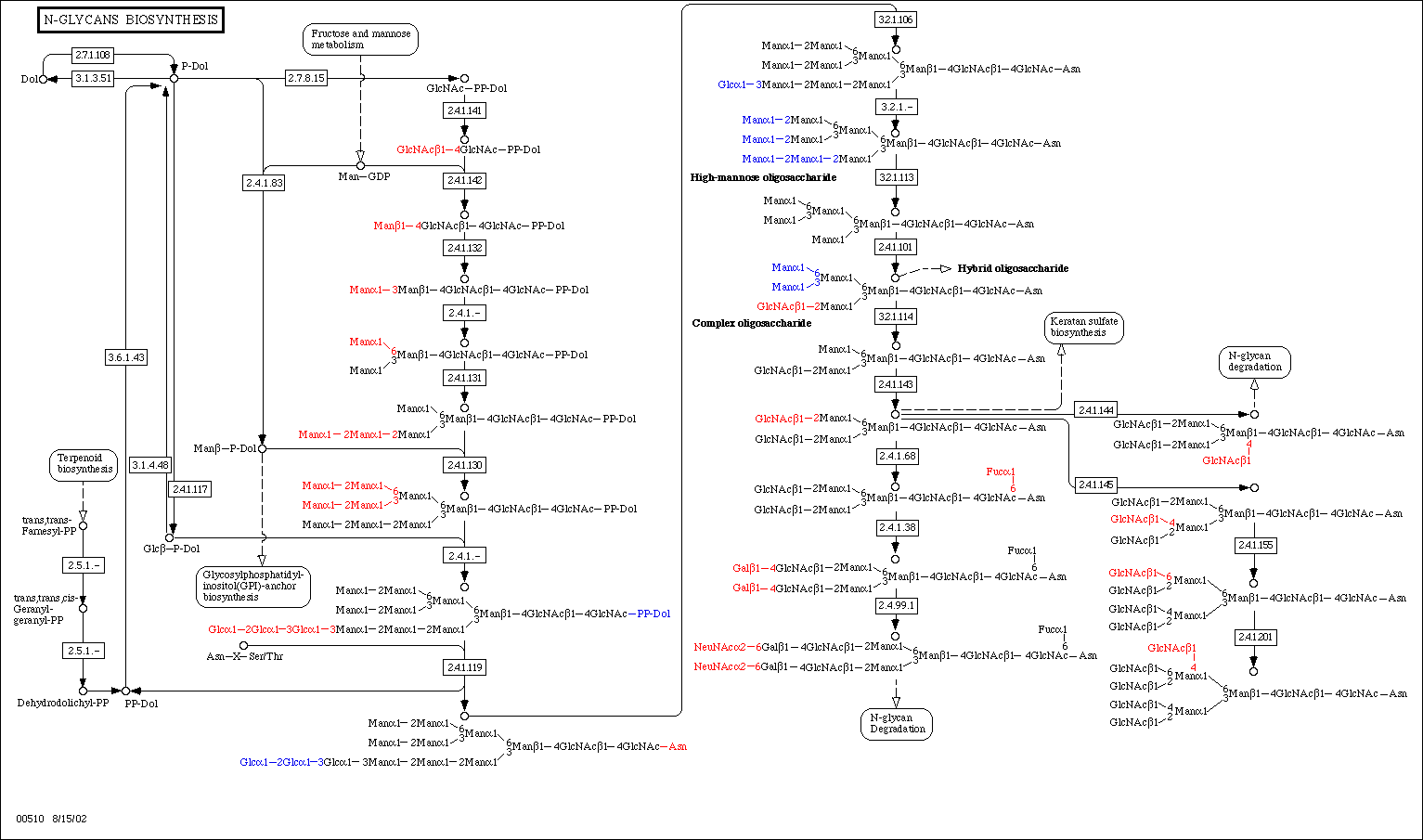
GnT-V protein is encoded by the gene MGAT5, which is located on chromosome two at position 2q21. GnT-V is a rate-limiting enzyme in the N-glycan pathway. The N-glycan pathway is used to modify originally similar glycoproteins on the outside of the cell. In addition to other Golgi enzymes such as glucosidases and mannosidases, N-acetylglucosamine (GlcNac) is glycosylated in the endoplasmic reticulum before being added to the outside of the cell. It is on this glycoprotein that GnT-V acts. GnT-V catalyzes the addition of 1,6-GlcNAc to form tri- and tetra-antenna-like oligosaccharides (Couldrey et al. 2000). All cells have this capability, but GnT-V activity is most often associated with normal T cell proliferation (Alverez et al. 2002). This is not a surprise, as T cells are vital to an organism’s immune system, which is primarily concerned with identifying antigens through binding of this oligosaccharide. Below is a look at the process of N-glycan biosynthesis.

N-Glycan biosynthesis, of which GnT-V takes part. Courtesy KEGG.
At the KEGG website, a look at N-glycan biosynthesis can be examined for select organisms. A comprehensive look at N-glycan degredation can be found here, at the Kyoto Encyclopedia of Genes and Genomes.
Chime Image of the crystal structure of N-acetylglucosaminyltransferase I, closely related to GnT-V. Courtesy PDB.
Cancer Associations:
GnT-V proves to be a double-edged sword for the human body. On the one hand, it appears to be essential for the function of the immune system. Presumably, because there have been no mutants reported, this enzyme is vital. However, at the onset of cancer, GnT-V proves to be problematic, as its activity increases and affects the cancerous cell’s motility (Xia et al. 1998 and Wang et al. 2001). Much research has gone into examining GnT-V’s actual function during oncogenesis and metastasis. First, it appears that during oncogenesis, branching of the GlcNAc oligosaccharides occurs (Guo, et al. 2002). This is primarily due to the cell’s change from performing its specific function to becoming a cancerous, reproducing cell without regulation – glycan biosynthesis is another process that gets overly induced as a result of this change. Physically, an overproduction of GnT-V increases the branching of ß1,6-GlcNAc (Guo et al. 2002), an oligosaccharide on the outside of the cell. Due to this increased branching, the cell is no longer able to hold onto the integrin subunits of the fibronectin receptor, which is responsible for holding the cell in its framework with other cells (Guo et al. 2002). When this mechanism breaks down, metastasis occurs and the cell can move along the bloodstream or lymphatic pathway and create a tumor elsewhere. This is almost always detrimental to the person under treatment, as recovery from treatment in multiple areas becomes more and more impossible. Using GnT-V as an indicator for a cancer’s motility may also prove useful for correct diagnosis and medical intervention (Yanagi, et al. 2001).
Orthologs, etc:
Upon the elucidation of the human genome, an ortholog to GnT-V was found that had 60% homology. This GnT-V is currently under examination by researchers. In addition to the alternate GnT-V sequence found in humans, this protein is found in some capacity in C. elegans on up the organism hierarchy. Obviously, then, GnT-V itself or a primitive precursor is responsible for something less specific than T-cell proliferation and function. In mouse, there is a 87% identity between the two forms of GnT-V, which can be found by performing a BLAST2 with the two proteins. To view human and mouse sequences, click here. Homologs are also found in R. norvegicus, C. griseus, and C. elegans. The gene that is homologous to MGAT5 in worm is called Gly2, and it has 36% identity with the human gene.
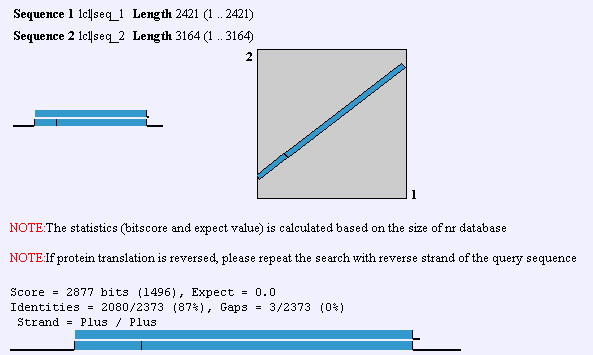
A Blast2 between human and mouse nucleotide sequences. Screen shot from NCBI.
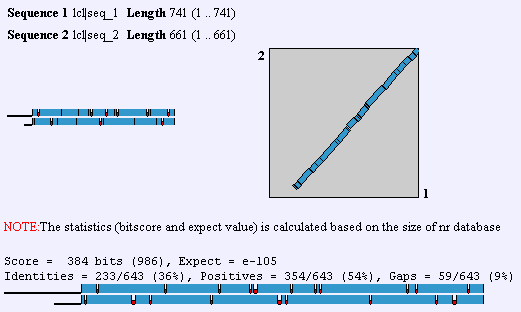
A Blast2 between human and worm (C. elegans) homologous proteins. Screen shot from NCBI.
Websites Used:
References:
Alverez K, Haswell C, St Clair M, Perng GS, Shorebah M, Pierce M, Fregien N. "Sequences of the mouse N-acetylglucosaminyltransferase V (Mgat5) mRNA and an mRNA expressed by an Mgat5-deficient cell line." Glycobiology 2002 Jul;12(7):389-94.*
Chakraborty AK, Pawelek J, Ikeda Y, Miyoshi E, Kolesnikova N, Funasaka Y, Ichihashi M, Taniguchi N. "Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, beta1-6 branching, and metastasis." Cell Growth Differ 2001 Dec;12(12):623-30.*
Chen, L, Zhang, W, Fregien, N, & Pierce, M. "The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyl transferase V and expression of its cell surface oligosaccharide products." Oncogene 1998, 17:20872093.
Couldrey C, Green JE. "Metastases: the glycan connection." Breast Cancer Research 2000;2(5):321-3.*
Cummings, RD, Trowbridge, IS, & Kornfeld, S. "A mouse lymphoma cell line resistant to the leukoaglutinating lectin from Phaseolus vulgaris is deficient in UDP-GlcNAc: alpha-D-mannoside beta-1,6-N-acetylglucosaminyltransferase." J Biol Chem 1982, 257:1342113427.
Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. "Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration." Cancer Research 2002 Dec 1;62(23):6837-45.
Wang LY, Chen KL, Su JM, Jin JW, Chen HL, Zha XL. "GnT-V overexpression in human hepatocarcinoma cells affects its migration and expression of cell adhesion molecules." Shi Yan Sheng Wu Xue Bao 2001 Sep;34(3):219-25.
Xia BY, Guo HB, Chen HL. "Dual Regulation of Tyrosine Protein Kinase and Protein Kinase C on N-acetylglucosaminyltransferase V." Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 1998;30(5):427-431.
Yanagi
M, Aoyagi Y, Suda T, Mita Y, Asakura H. "N-Acetylglucosaminyltransferase
V as a possible aid for the evaluation of tumor invasiveness in patients with
hepatocellular carcinoma." Journal of Gastroenterol Hepatology
2001 Nov;16(11):1282-9.
© Copyright 2003 Department of Biology, Davidson College, Davidson,
NC 28035
Send comments, questions, and suggestions to: dapierce@davidson.edu