This web page was made as an assignment for an undergraduate course at Davidson
College.
My Favorite Protein:
Erythropoietin
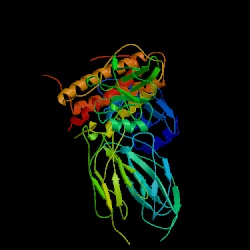
Figure 1. Structure of EPO complexed
with extracellular domainans of EPO receptor shown in ribbons. Image taken
from PDI. Click on Image to find out more about its source. Permission
Pending
What is Erythropoietin?
Erythropoietin, also known as EPO is acidic glycoprotein growth factor that
triggers erythrocyte, or red blood cell production (Erslev 1991). The 5 exons
of the EPO gene encodes 193 amino acids, 27 of which are later cleaved off to
produce a 166 amino acid long peptide although the circulating peptide contains
165 amino acids. The mechanism for this is cleavage is unknown (OMIM
2003). EPO is produced by the kidney or liver of adult mammals and also
produced by the liver of fetal and neonatal mammals (Genecards
2003).
How does Erythropoietin control erythrocyte concentration?
Erythropoietin triggers the production of erythrocytes that make up the majority
of the cells within blood. The purpose of red blood cells is to transport respiratory
gases. Low levels of oxygen levels in the body, known as hypoxia, causes the
pathway leading to EPO production, and consequentially, erythrocyte production.
This process is done through the use of the transcription factor, HIF-1 which
many tissues give off in reduced oxygen conditions. HIF- 1 tells the kidney
(or liver) to produce EPO. EPO then binds to two receptors (EPO- R) found on
stem cells in the bone marrow of ribs, breastbone, pelvis and vertebrae. This
leads to the maturation to functional red blood cells, and ultimately the increase
of oxygen supply in tissues (Purves et al. 2001). Thus, when EPO is present,
red blood cells mature. When EPO is unavailable, they die (Erslev 1991).
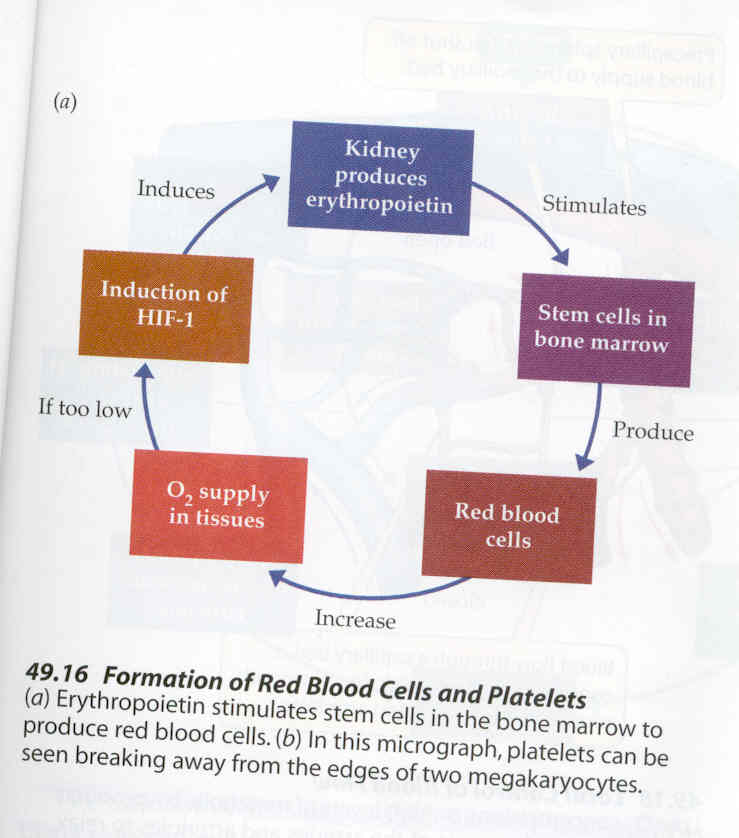
Figure 2. Figure from Life: The Science of Biology,
Sixth Edition (Purves et al. 2001). This figure demostrates how low oxygen levels
cause the growth factor HIF-1 to then trigger the kidney to make erythropoietin.
EPO then causes stem cells to synthesize red blood cells which cause the oxygen
supply within tissues to become greater. Permission Pending.
Structure of Erythropoietin
Figure 3. Figure
from PDB.Click here to download Chime image.. Chime image of Erythropoietin.
Click to Rotate Protein.
Erythropoietin is composed of an "up-up- down-down four helical bundle
topology" and has "two small antiparallel beta strands typical of
the short- chain class" (Syed et al. 1998). A disulphide bridge connects
one of the pairs of antiparallel long helices from Cys 7 to Cys 161, while another
antiparallel long helix (between alpha B and alpha C regions) is connected by
a short loop. An irregularity at Gly 151 results in a kink in the alpha D helix.
A beta sheet also results from amino acids of the AB and CD crossover loops.
A, B, and C helices combined with many aromatic and hydrophobic regions form
the interior of EPO. In addition, short helices exist near both alpha B and
alpha C regions (Syed et al. 1998). EPO binds to two receptor proteins (EPObp2
and EPObp1), and thus has two binding sites (Syed et al. 1998).
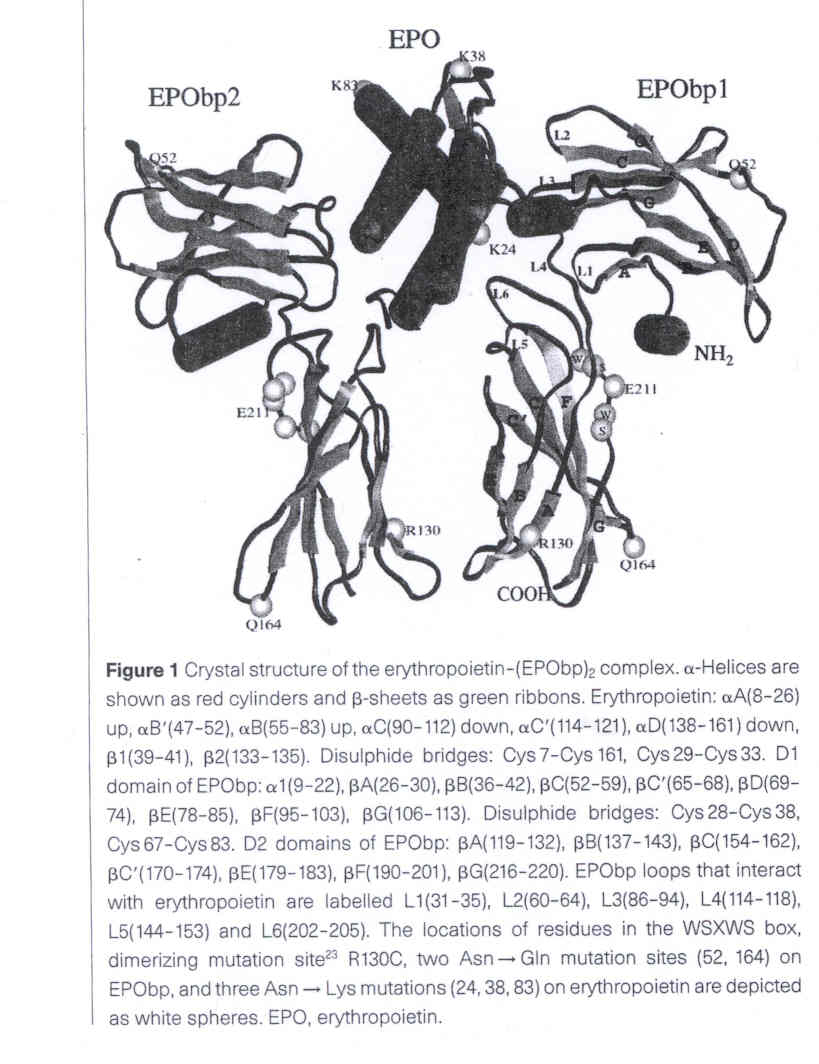
Figure 4. Figure from Syed et al. 2003. Figure shows the Crystal
structure of the erythropoietin complexed with its two receptors, EPObp2 and
EPO bp1. Alpha helices are shown as cylinders while beta sheets are shown as
ribbons. Permission Pending.
Mutants of Erythropoietin
Since Gly 151 in the D helix of erythropoietin connects the side chain of Lys
152 into hydropobic contact with Val 63, Trp 51, and Phe 148 within the interior
of the protein, the replacement of alanine at either position 151 or 152 would
cause a loss of activity. Mutations to acidic amino acids do cause a considerable
loss of reactivity although substitutions at the basic positions of Lys 20 and
Lys 45 result in no loss of bioactivity. Two different amino acid positions
that naturally contain Arg are very susceptible to mutations that result in
loss of bioactivity. These two sites are Arg103 (that results in mutant R103A)
and Arg 14 (that results in mutant R14Q). Both Arg 103 and Arg 14 are involved
in site 2 binding, but a mutation in Arg 103 only results in loss of site 2
binding, whereas a mutation in Arg 14 results in an overall fivefold loss in
affinity (for both binding sites 1 and 2) (Syed et al. 1998).
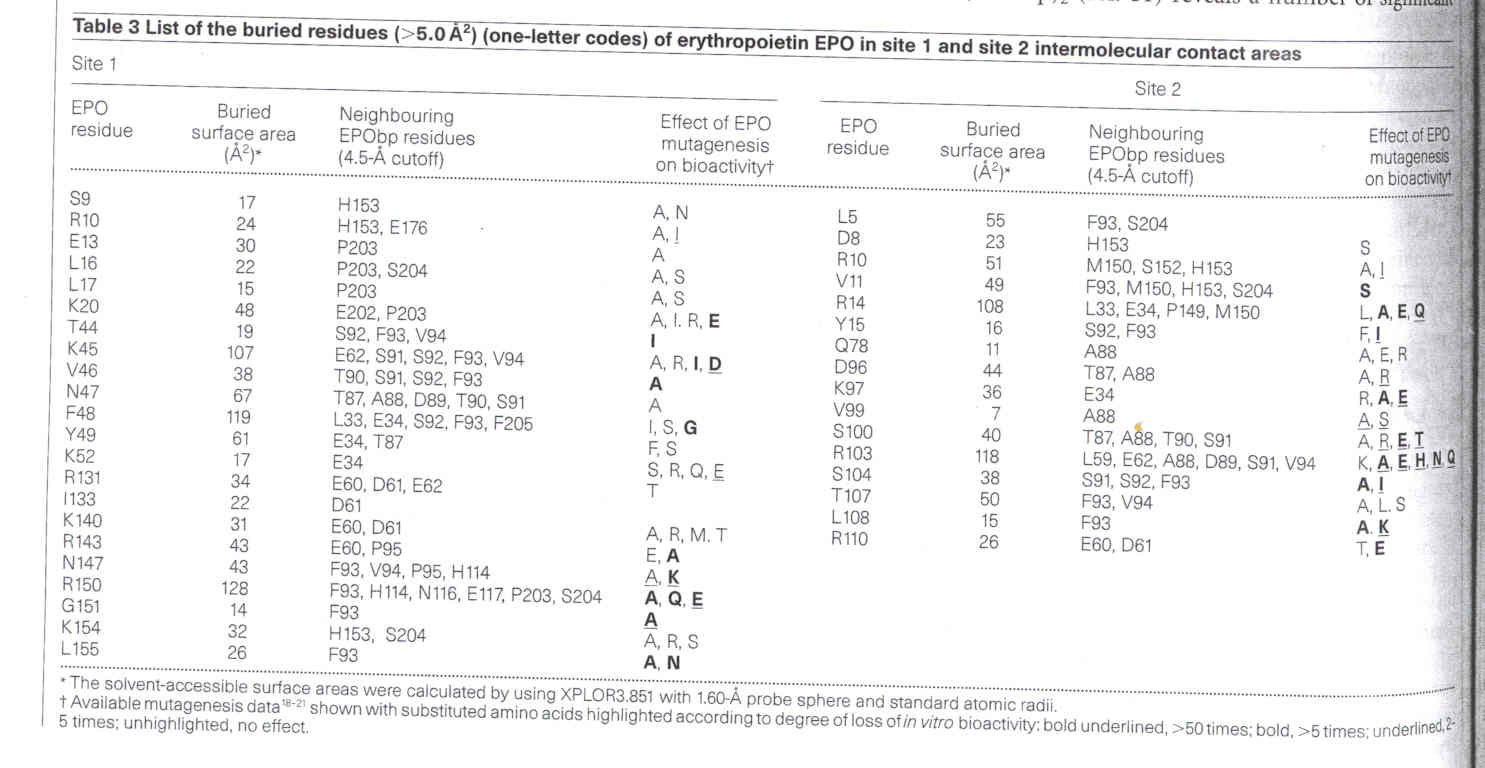
Table 1: Table from Nature (Syed et al.). This table shows
the amino acid residues that are within the functional eptitope of erythropoietin.
Mutations that will cause the most change in bioactivity are shown and the degree
to which they cause loss of in vitro bioactivity is marked (bold and
underlined, > 50 times; bold, >5 times; underlined, 2-5 times; unhighlighted,
no effect).
Erythropoietin and Disease
The result of the underproduction of EPO is linked to a condition known as
anemia, or the exhaustion of red blood cells (Purves et al. 2001). Among some
of the diseases associated or coincide with underproduction of EPO are cancer,
rheumatoid arthritis, HIV infection, sickle cell anemia, and anemia of prematurity.
In some of these cases, like anemia of prematurity, a problem within the translation
of the erythropoietin- coding gene into its protein is the cause of low EPO
levels (Faruki and
Kiss 1995). Anemia of prematurity seems to be caused by this underproduction
of EPO. It is believed that the switch from the synthesis of erythropoietin
in the liver to synthesis within the kidney that happens at birth in many mammals
may be the cause of underproduction of EPO in premature infants. There is believed
to be a delay in the switch to renal EPO synthesis, and so less erythropoietin
is produced in premature babies. In other diseases, such as chronic renal disease,
the decrease in EPO production is due to the fact that the kidney’s function
is impaired, and likewise, because erythropoietin is produced mostly in the
kidneys, its production is impaired also (Erslev 1991). However, in cases of
anemia associated with cancer and other chronic diseases, the cause of decreased
levels of EPO are due to the inhibition of EPO by inflammatory cytokines such
as IL-1 and TNF that are generated in the presence of these diseases (Faruki
and Kiss 1995).
Treatment of Anemia
Anemia caused by low levels of EPO can be treated through the use of recombinant
EPO or rhu- EPO. The gene encoding EPO was abstracted, spliced into an expression
vector and multiplied through the use of bacteria. Because people who have kidney
failure undergo dialysis that removes toxins, and in the process EPO from their
body, they lack whatever EPO their body did make. Recombinant EPO thus given
to patients undergoing dialysis to restore their EPO levels (Purves et al. 2001)
Human Erythropoietin Amino Acid Sequence and Orthologs
For
Homo Sapiens
For
Mus musculus (house mouse)
For
Equus callabus (horse)
For
Bos taurus (cow)
Related sites:
http://bioinfo.weizmann.ac.il/cards-bin/carddisp?EPO&search=erythropoietin&suff=txt
http://www.bcm.tmc.edu/medicine/hema-onco/lectures/Erythropoiesis.html
http://www.clunet.edu/BioDev/omm/epo/frames/epotxt.htm
References:
Erslev AJ. 1991. Erythropotein. New England Journal of Medicine 324: 1339-1344.
Faruki H, Kiss JE. 1995 July. Erythropoietin. The Institute for Transfusion
Medicine. <http://path.upmc.edu/consult/rla/july1995.html>
Accessed 2003 Mar 10.
GeneCards. 1997-2001. GeneCard for gene EPO GC07P098853. Weizmann Institute
of Science. <http://bioinfo.weizmann.ac.il/cards-bin/carddisp?EPO&search=erythropoietin&suff=txt>
Accessed 2003 Mar 11.
McKusick VA. 1986 June 4. *133170 Erythropoietin, EPO. OMIM. <http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=133170>
Accessed 2003 Mar 10.
NCBI. Nation Center for Biotechnology Information. Individual links found with
Information.
Purves WK, Sadava D, Orians GH, Heller HC. 2001. Life: the Science of Biology,
Sixth Edition. Sunderland, Massachusetts: Sinauer Associates, Inc, pp:324-325
and 879-881.
Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino
AJ, Zhang J, Finer- Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski
JJ, Egrie J, Stroud, RM. 1998. Effieciency of Signalling through cytokine receptors
depends critically on receptor orientation. Nature 395: 511-516.
Molecular
Biology Main Page
Course
Materials
Biology Main Page


Questions? E-mail Holly Smith at hosmith@davidson.edu



