My Favorite Gene/Protein:
*This page was produced as part of an undergraduate course at Davidson College*
FBN-1/Fibrillin
Overview-
Fibrillin was identified in 1986 when monoclonal antibodies specific for fibrillin were used to identify the protein in the extracellular matrix of skin, lungs, cartilage, and vascular tissue among other major tissues (Sakei et al., 1986). More specifically, it is found in the microfibrils of the extracellular matrix. It has a weight of roughly 350,000 Da. and encodes a protein that is 2,871 amino acids long with five distinct regions including some distinctive cysteine-rich domains and calcium binding domains. Between these domains are tandem repeats of an EGF sequence. The fibrillin producing gene is roughly 65 exons in length (Corson et al., 1993) and is located on chromosome 15. The FBN-1 gene itself is estimated to be roughly 200kb in length. The exact role of fibrillin within the extracellular connective tissue is unknown mainly due to the lack of information on the actual mechanical role of microfibrils. However, it is known that it is a major protein construction block for this ever important tissue with its capacity for elasticism and its ability to bind calcium and thus facilitate protein-protein interaction.
For a look at the sequence of the FBN-1 gene have a look here.

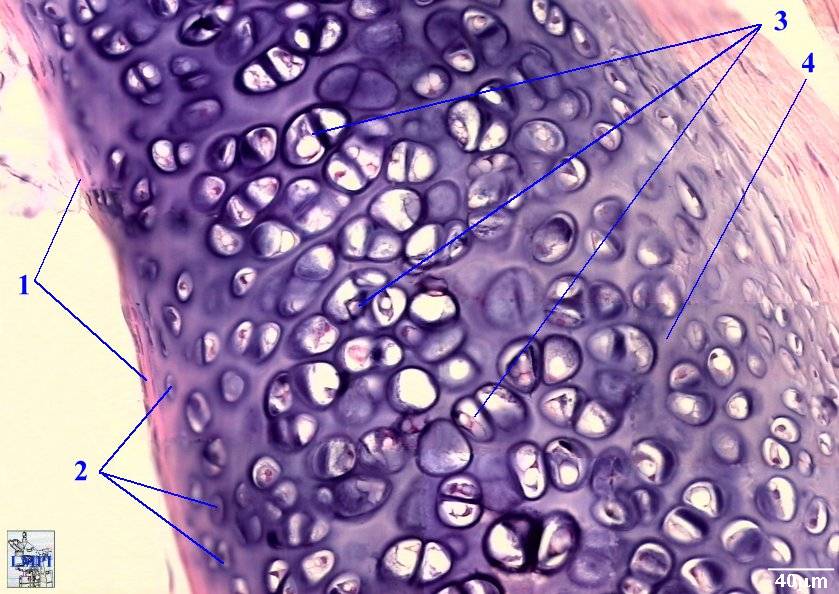
*Image permission pending from the Digital Atlas of Histology*
Figure 1- An image of cartilage. The vast interconnective ECM is indicated by the number 4 on the figure in which fibrillin is a major protein building block.
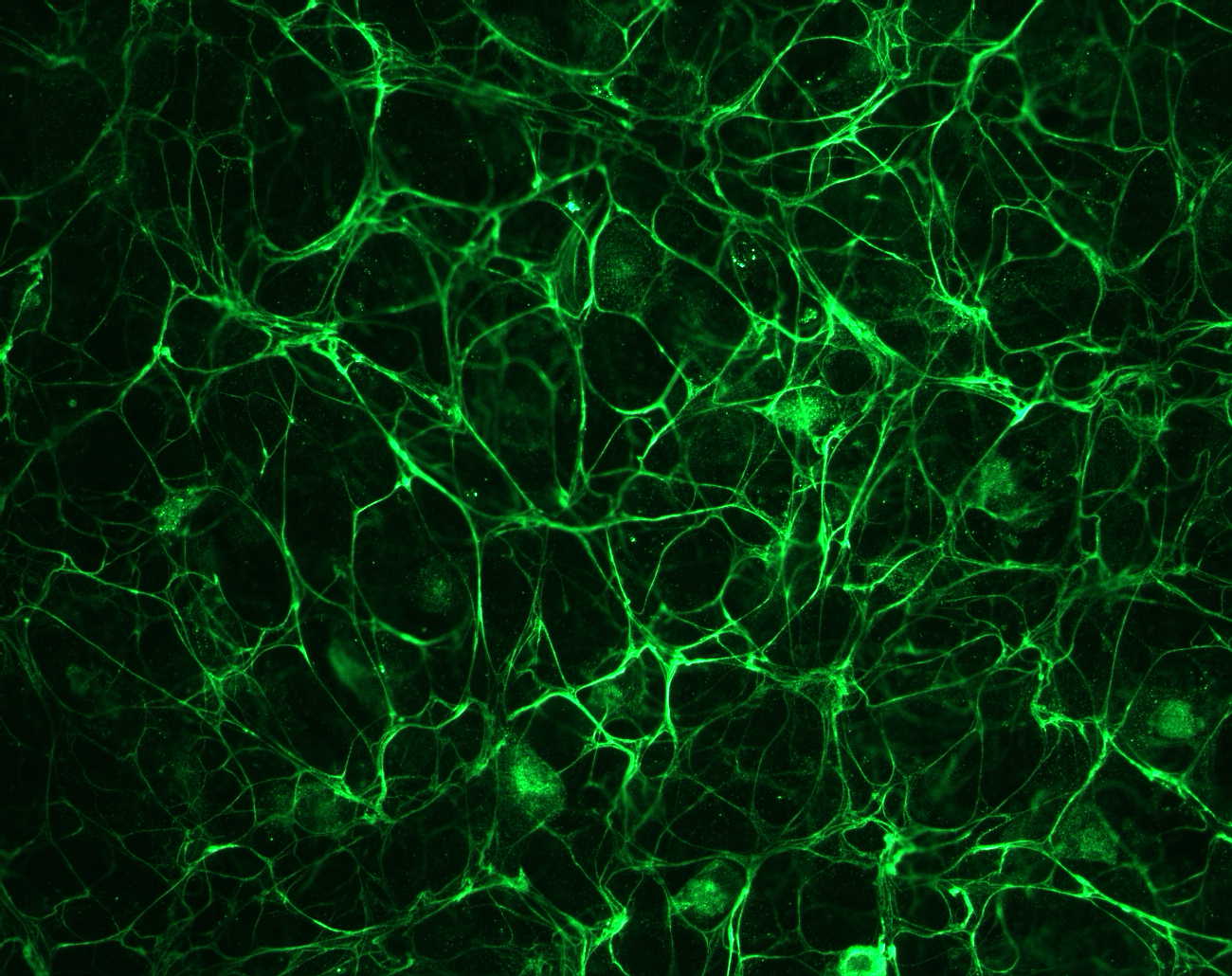
Figure 2- An image of pigmented epithelium cells stained with a fibrillin antibody from Dr. Rettinger at http://ef.wustl.edu/genes/FBN1.htm#short%20description
Structure-
The FBN-1 gene encodes a large cysteine-rich and multi domain glycoprotein with extensive intrachain disulfide bonds with all the cysteine-rich repeats being encoded by single exons. Its organization includes 43 calcium binding epidermal growth factor-like domains and several growth factor-like domains that are cysteine rich. It is also possessed of several N-linked glycosylation sites that are interspersed randomly throughout the protein.
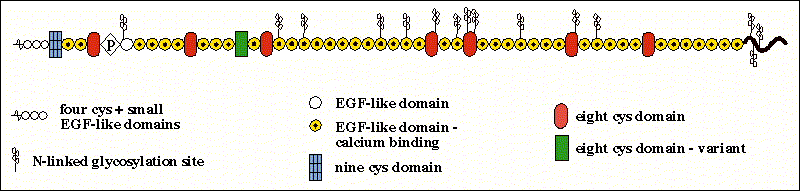
Figure 3- An image of the rough structure of the fibrillin protein indicating the different domains from Dr. Rettinger at http://ef.wustl.edu/genes/FBN1.htm#short%20description*.
Cross-Species Analysis-
The provided links are to the National Center for BioTechnology Information and give sequence information along with many other tidbits about a given protein.
FBN-1 and orthologs found in many different species of animal possessing the same role:
Jellyfish The protein is similar to fibrillin but it changes with the stage of life of the jellyfish.
Mouse Mouse Fibrillin
Chicken Chicken Firbrillin
Marfans Syndrome-
Marfans Syndrome is an autosomal dominant disease possessing a dominant negative protein product effect that affects the microfibrils of the extracellular matrix. It has been linked to several different missense mutations (some that affect calcium binding), nonsense mutations resulting in an exon skip or the production of a stop codon, and even a frame shift mutation. The very first mutation ever discovered was R1137P in 1991 and then built upon to discover four other novel mutations in 1993.
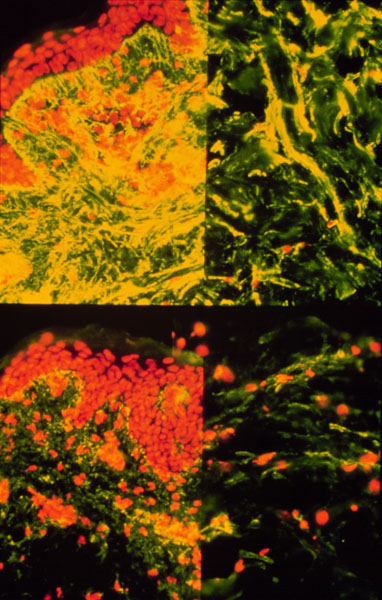
*Image permission pending from Dr. Simpson*
Figure 4- Histological diagram of fibrillin and other molecules in ECM tissue. Fibrillin is stained with yellow. Notice the disparity in fibrillin count between the normal patient at the top and the Marfans patient at the bottom.

*Image permission pending from Dr. Simpson*
Figure 5- Image depicting exons in which Marfans causing mutations have been located, there are several.
Clinical Signs of Marfans Syndrome-
- Concave Chest
- Arachnodactyly
- Elongated arms in comparison to height
- Sculliosis
- Ocular and Facial Deformity
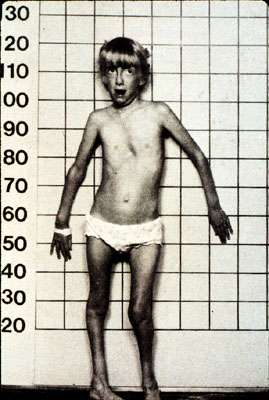
*Image permission pending from Dr. Simpson*
Figure 6- A young girl with most of the classic signs and a diagnosis of Marfans Syndrome.
Marfans is not inherently deadly in and of itself and is manageable clinically by treating the one deadly side effect of this syndrome. The actual mechanics of the syndrome are poorly understood. It is a common cause of death for a Marfans subject to experience an aortic aneurysm or rupture due to the ballooning and thus stretching of the vascular extracellular matrix due to the fibrillin mutation. The rupture can occur as either a weak spot in the vascular tissue or as a dissection, which is when blood enters the lining of the aorta and splits it in two. Thus, clinical treatments include beta blockers and other vascular aids. Olympic athlete Flo Hyman (follow the link for an article about her career and death) passed away from exactly such a rupture after extreme exertion at a routine tournament game.
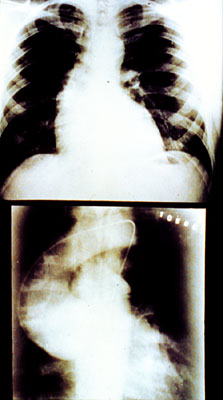
*Image permission pending from Dr. Simpson*
Figure 7- An x-ray of an aortic aneurysm.
SHPRINTZEN-GOLDBERG CRANIOSYNOSTOSIS SYNDROME-
This disease is virtually identical to Marfans Syndrome in its symptoms but this syndrome refers to the tendency of the skull in those with a mutation in the fibrillin-1 gene to prematurely close thus causing structural abnormalities such as a cloverleaf shape. The disease is autosomal dominant in transmission and usually results from a missense mutation at one of many sights such as C1223Y or P1148A.
References:
Sakai et al (1986).
"Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils."
J. Cell Biol. 103: 2499-2509
Questions? Email the webmaster at matalbert@davidson.edu!
Want to go to the Davidson Molecular Biology Homepage? Click here.