 gggggggggggggggggggggggg
gggggggggggggggggggggggg![]() d2.
d
d2.
d![]()
This web page was produced as an assignment for an undergraduate course at Davidson College.
Affinity Chromatography:
An in-Depth Look
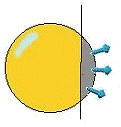
The main idea behind affinity chromatography is that this technique makes use of a specific binding site to a target biomolecule that can be used to pull it away from all the others in a solution. Figure 1 gives a representation of how a matrix with binding sites might look, with figure 2 and 3 representing the individual binding molecules.
 gggggggggggggggggggggggg
gggggggggggggggggggggggg![]() d2.
d
d2.
d![]()
© Amersham Biosciences Limited - All rights reservedggggmmmgggFig 2.dddFig 3.
Fig.1. Representation of a matrix with binding sites. Fig 2. represents the actual binding sites of the matrix, while Fig 3. represents the enzyme that is trying to be isolate
Affinity chromatography can be looked at as a system, made up of many key parts. However, affinity chromatography is mainly made up of three main steps: equilibration, sample application and wash and elution (Amersham Bioscience, 2003). Equilibration is the first step and involves pretreating the binding solution with a binding buffer to equilibrate the matrix and prepare it for adsorption. (shown in Figure 4.) The matrix is basically any substance that the need to be isolated biomolecule will bind to along with unbinding to. A matrix is one of the most important parts of the AC system because it composes, for the most part, the largest part of the absorbent (Scouten, 1981). Because of this, the matrix must have several essential properties. (see Table 1).
© Amersham Biosciences Limited - All rights reserved
Fig.4. Equilibration gHHH
Table 1. Ideal Matrix Properties (Scouten, 1981)
Easily deviatized
Both mechanically and chemically stable
Possess surfaces with easy ligand accessibility
Good flow characteristics
Stable to eluant, e.g., denaturing buffers
Most matrices employed in today's workforce, can be divided into two main groups; first generation matrices that are mainly single-composition matrices and second-generation matrices. Some examples of first generation matrices are agarose, glass, or cellulose, while examples of second-generation matrices would be dual-composition and/or chemically modified matrices such as glycidoxy-coated glass, agarose-coated polyacrylamide (Utragel) and polyacrylic-coated iron particles (Enzacryl) (Scouten, 1981). As of now, most of the matrices used for AC are still the first generation matrices, but the newer matrices offer new possibilities for improvement of affinity systems, due to there combination of the best of two matrices properties (Scouten, 1981). Sometimes, though, even though one has selected a matrix with the right chemical properties for his biomolecule, the matrix's bound ligands need a little help. This help comes in the form of spacer arm (Figure 5.)
dddddddddd
© Amersham Biosciences Limited - All rights reserved
Fig. 5. Matrix with bound ligand showing the use of ddddFig. 6. Sample application and wash
spacer arm to increase distance from sufacedddddddddddddddddddddddddddd
A spacer arm is used when the biomolecules receptor area is too large for the affinity ligand. By adding a spacer arm, the affinity ligand is moved off the surface of the matrix, allowing for the biomolecule to bind. After one has chosen and prepared their matrix, the next step is to add the sample and then wash the matrix (Figure 6). The main goal of this step is to get the wanted biomolecule to bind to the ligand and then to wash the unwanted and unbound molecules away. Hopefully, if one has chosen the right ligand the biomolecule will bind reversibly to the ligand and the non-binding molecules will simply pass through during washing (Amersham Bioscience, 2003). The next and final step in affinity chromatography is elution or the disassociation of the biomolecule from the ligand (Figure 7).
© Amersham Biosciences Limited - All rights reserved
Fig. 7. Elution
Through the addition of an elution buffer, the biomolecule is released from its chemical bond with the ligand. This can be accomplished in many different ways, but the most common are by changing the conditions of the solution (either by pH, temperature or ionic strength), through the addition of a free ligand or through the addition of a competitor. By changing the conditions of the solution that the ligand and biomolecule are binding in, one can make the energy of the bond no longer great enough to keep the bond intact. Addition of a free ligand and addition of a competitor work not on the solution, however, but on the actual physical molecules in the solution: the ligand and the bound biomolecule. The addition of a free ligand works by challenging the matrix bound ligand for the biomolecule. If the free ligand forms a stronger bond with the biomolecule, then the matrix-bound ligand will unbind with the biomolecule and the free ligand will take the matrix-bound ligands place with the biomolecule (Amersham Bioscience, 2003). This will allow the user to then wash free the free ligand and the biomolecule (Figure 8).
HHHHHdddddddddddddddddddddH
© Amersham Biosciences Limited - All rights reserved
ddddddFig. 8. Through the addition of a free ligand the free ligandddddddFig. 9. Competitor shown out binding the
dd ddDout binds the matrix-bound ligand for the biomolecule ddddddddtrbiomolecule for the matrix-bound ligand.The addition of a competitor (Figure 9) works almost exactly like the free ligand, but instead of binding to the biomolecule, the competitor binds to the matrix-bound ligand (Amersham Bioscience, 2003). By having a higher affinity for the matrix-bound ligand then the target biomolecule, the competitor replaces the target molecule and binds to the bound ligand. This, then allows for the target molecule to be washed free for further analysis.
_______________________________________________________________________________________
_________________________________________________________________________________________
© Copyright 2003 Department of Biology, Davidson College, Davidson, NC
28035
Send comments, questions, and suggestions to: wiwood@davidson.edu