This web page was produced as an assignment for an undergraduate course at Davidson College.
ATP Synthase
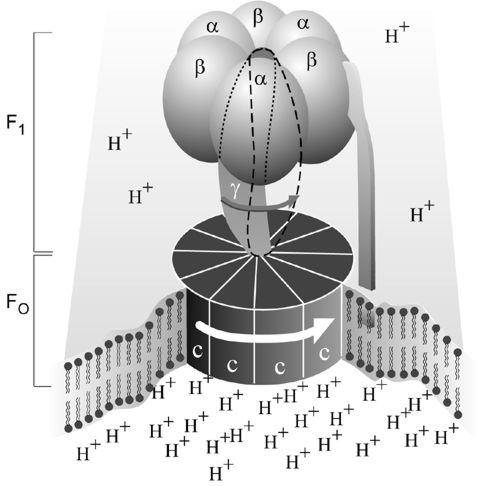
Fig. 1. Simplified picture of ATP Synthase. Note the F1 and Fo regions. Illustration provided by Nobelprize.org.
Background:
ATP Synthase is considered to be the universal carrier of chemical energy in the cell. It captures the energy released by the combustion of nutrients and then uses that energy to fuel other reactions that require it. Most ATP is formed in the mitochondria during cellular respiration or in the cytoplasm during photosynthesis. ATP Synthesis was originally isolated from the mitochondria (Walker et al, 1990).
In most systems, the ATP synthase sits in the membrane (the "coupling" membrane), and catalyses the synthesis of ATP from ADP and phosphate driven by a flux of protons across the membrane down the proton gradient generated by electron transfer. The flux goes from the protochemically positive (P) side (high proton electrochemical potential) to the protochemically negative (N) side. The reaction catalyzed by ATP synthase is fully reversible, so ATP hydrolysis generates a proton gradient by a reversal of this flux. In some bacteria, the main function is to operate in the ATP hydrolysis direction, using ATP generated by fermentative metabolism to provide a proton gradient to drive substrate accumulation, and maintain ionic balance.
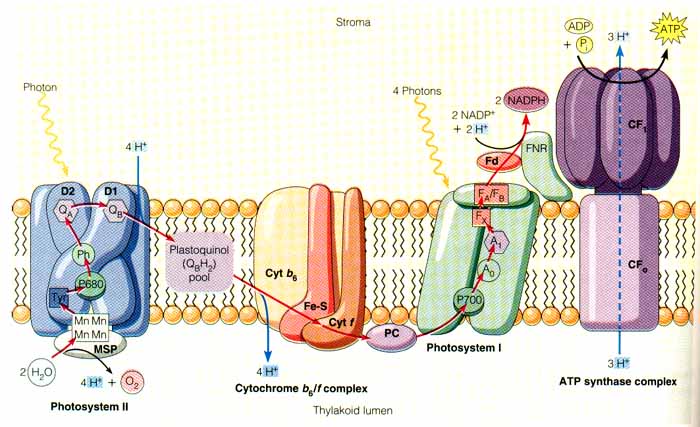
Fig 2. Light Driven ATP Synthesis. This figure illustrates one important function of ATP Synthase. Image provided by winona.edu.
Structure and Function:
ATP Synthase is made up of two main parts: F1 and Fo. F1 contains the catalytic center, and Fo couples F1 to the membrane and is in charge of transporting hydrogen ions. Fo has three types of subunits: proteins 'a', 'b', and 'c'. Of these, Fo has one 'a', two 'b's, and nine-twelve 'c's (Walker et al, 1990). F1 has five subunits: three alphas, three betas, one gamma, one, delta, and one epsilon. The beta subunit is where synthesis of ATP actually occurs. The gamma, delta, and epsilon subunits are not symmetrical. This becomes important in discussing the function of ATP Synthase and it's parts.
In their nobel prize winning work on ATP Synthase structure, Boyer and Walker (Royal Swedish Academy, 1997) found that instead of the synthesis of ADP to ATP requiring energy, it was the binding of ADP to and the phosphate to the enzyme and the release of ATP that actually required the energy. Enzymes typically bind and release substrates spontaneously (Walker et al, 1990), for this, the overall reaction will require energy. This is how ATP Synthase differs from many enzymes. Here, the asymetrical structure comes into play. Each of the three beta subunits has different couplings to the gamma, delta, and epsilon subunits, yet they function in the exact same way. Boyer discovered that gamma, delta, and epsilon each opperate in a cylinder formation that is comprised of alpha and beta subunits (Royal Swedish Academy, 1997). The rotation of these cylinders is hypothesized to be caused by the hydrogen ion flow across the membrane (Walker et al, 1990). Figure 3 illustrates Boyer's discovery, which is now coined "Binding Change Mechanism". The rotation of alpha and beta subunits causes structural changes in the enzyme which lead to functional changes. With each rotation, the binding ability of the F1 part of the enzyme changes. The interaction of the gamma subunit with the alphas and betas forces "their active surfaces to assume different three-dimmensional structures" (Walker et al, 1990).
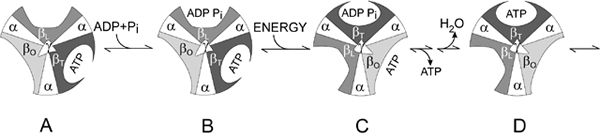
Fig. 3. Boyer's "Binding Change Mechanism". Each cylinder represents a different stage of ATP synthesis. Illustration provided by Nobelprize.org.
Walker verified Boyer's predicted mechanism by determining the crystellographic structure of the F1 part of bovine ATP Synthase. He found that the alpha and beta subunits have very different structures and therefore have different ways or abilities of binding ATP and ADP. The amino acid sequence of ATP Synthase has directly provided valuable information regarding its function. Through the amino acids already sequenced, a basis for the secondary structure of components of the transmembrane proton channel has been established (Walker et al, 1990). Also, scientists have been able to identify amino acids that are important to the nucleotide binding sites using the already sequenced amino acids in ATP Synthase.
To find out more about ATP Synthase and its orthologs, click HERE.
Related Sites:
Animation Movies of ATP Synthase
Biological Energy Conservation
References:
Ko, Young Hee, et al. 2000. Mitochondria FoF1 ATP Synthase. JBC Papers in Press 275, 32931-32939.
Royal Swedish Academy of Sciences. 1997. Press Release: The 1997 Nobel Prize in Chemistry. <Nobelprize.org>
Walker, J.E., et al. 1990. Structural Aspects of Proton-Pumping ATPases. Philosophical Transactions of the Royal Society of London 326, 367-378.
Walker, J.E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621-628.
© Copyright 2002 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: jecarlson@davidson.edu