*This website was produced as an assignment for an undergraduate course at Davidson College.*
EGFR Tyrosine Kinase Orthologs
What are Orthologs?
Orthologs are genes that are found in different species that, through speciation, have evolved from the same gene in an ancestor. The orthologs more often than not retain the same function, but this is not always true. By comparing the amino acid sequences between the orthologs, it is possible to determine which parts are conserved through speciation and which parts are "new". Such data is used to determine which amino acid sequences are important for the structure or function of the protein.
Review:
EGFR stands for Epidermal Growth Factor Receptor, and it is a receptor molecule that binds with its ligand, EGF, to act as a cell signaling molecule. EGFR is a tyrosine kinase receptor which plays a crucial role in cell proliferation, tissue development, and division. The protein contains 3 regions, a cytoplasmic portion, membrane portion, and extracellular portion where the binding site is located. In the extracellular domain in mammals, there are two cystein rich regions. When activated, the protein transfers phosphate groups to tyrosine residues on specific proteins, which normally results in the proteins activation, causing it to signal another mechanism within the cell. For a full review, click here.
EGFR Orthologs:

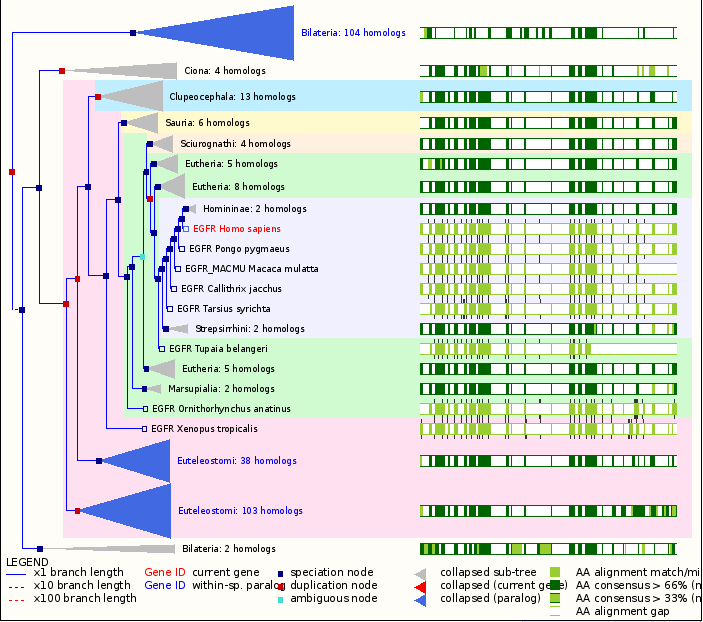
Figure 1: A gene tree of the EGFR protein. This gene tree shows the conservation of the EGFR amino acid sequence and its range of homologs.The high conservation indicates the proteins importance and stability. Image courtesy of ensembl.org.
The EGFR gene has been isolated in many different animals. This is primarily due to the necessity of the protein that it codes for. Because the protein is activated by extracellular signals (i.e. EGF) for cell to cell communication (Woodgett, 1994), it is found only in Animalia, or metazoans, which are multicellular. The EGFR gene and its orthologs are found in all vertebrate and the protein is highly conserved through nematodes to humans (Ghandi, 2006). The cystein rich regions are the most conserve regions in the extracellular domain of the EGFR tyrosine kinase. In the cytoplasmic domain, there are two highly conserved regions: the juxtamembrane region (for feedback control) and the tyrosine kinase region (for mediation of the six tyrosine autophosphorylation sites) (Bogdan, 2001).
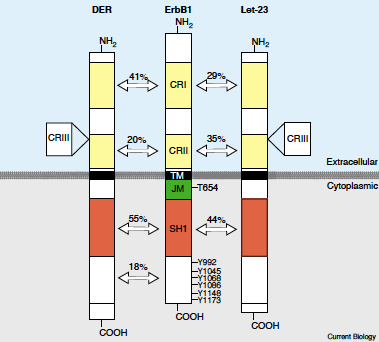
Figure 2: The EGFR structure and the amino acid percent identity between Drosophila EGFR, Human EGFR, and C.elegans EGFR. CRI and CRII are the Cystein rich regions, SH1 is the tyrosine kinase region, and JM is the juxtamembrane region. Image from Bogdan 2001. Permission pending.
Of the many animals studied for EGFR function and evolutionary relationship, C. elegans, Drosophila, and Xiphophorous seem to be the most studied. In C. elegans (nematode), the EGFR ortholog is LET–23. LET-23 produces an EGFR–like protein that contains three instead of two cystein rich regions (Bogdan, 2001), and all the three domains that human EGFR has. The resulting protein has a different function in this organism than that found in humans, as it plays a large role in the development of the organism. For example, LET-23 is crucial in ovulation, male spicule (copulatory spike-like organ) formation, and epidermal development (Ensembl.org).
In Drosophila, the EGFR ortholog is DER, and according to Bogdan, this gene has made Drosophila a powerful system for identifying new components which can inhibit EGFR. The DER protein is extremely similar to a human and mouse EGFR protein, however, it contains an extra 166 amino acids (Shilo, 1986). It too contains three cystein rich regions instead of two, as well as the typical transmembrane domain, extracellular domain, and cytoplasmic domain (Livneh, 1985)(Bogdan, 2001). In relation to the human EGFR, the drosophila receptor has 41% amino acid identity in the extracellular domain, and in the cytoplasmic domain has 55% identity (Livneh, 1985).The DER mRNA appears to have multiple splicing alternatives, and they are all found in tissue regions that generally proliferate, or require growth embryonically, leading to the idea that DER may have a crucial role in development (Shilo, 1986). This claim has been further studied by Schejter, who concluded that DER mutants have a "complex phenotype" that includes collapse of the nervous system and the deterioration of the organisms anterior structures, making them seemingly critical to development. In addition to binding with its ligands, it is capable of interacting with other membrane bound proteins, similar to human EGFR.

Figure 3: Clustal multiple sequence alignment between Mus musculus (mouse), Homo Sapien (human), Danio Rerio (zebrafish), Drosophila melanogaster (fruit fly), Caenorhabditis elegans (nematode). The EGFR-tyrosine kinase is conserved between all organisms. The (*) mean that all amino acids in the column are the same. (:)means that there are conservative substitutions. (.)means that there are slightly conservative substitutions. The colors have to do with specific properties of each amino acid.
According to Gomez, fish are now regarded as valuable organisms to study the function, development, and malfunction of molecules. As a result, Xiphophorous are beginning to be studied for their EGFR orthologs. In this fish, three EGFR related genes have been found, EGFRa, EGFRb, and Xmrc (Gomez, 266). EGFRa contains all the similarities of a receptor tyrosine kinase (2 cystein rich domains, extracellular portion, membrane-spanning region, cytoplasmic portion, and ATP binding portions) (Gomez, 2004). In EGFRa the tyrosine kinase domain is the most conserved (Gomez, 2004). The Xiphophorous EGFRb is a proto-oncogene, which means that it can be altered to produce a mutated EGFR which can result in a overexpression that can cause melanoma in fish (Gomez, 2004). The protein, Xmrc is extremely similar to that of the human EGFR protein, and it is an oncogene (Gomez, 2004). All of these EGFR genes have been found in other ray-finned lobe fishes, indicating that they are a result of evolutionary divergence.
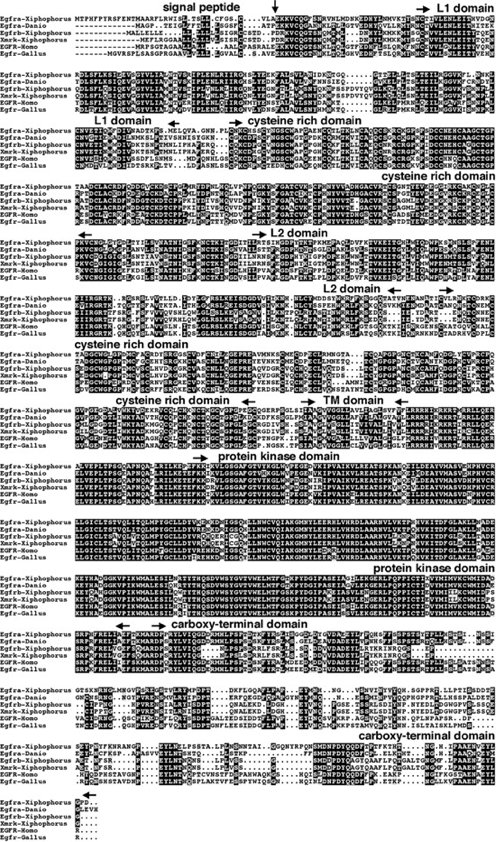
Figure 4: The amino acid sequence of the three different EGFR orthologs found in Xiphosphorous in comparison to humans, and chicken EGFR. The conserved amino acid sequences are in black boxes. The beginning and end of each conserved domain is indicated by the black arrow. Image from Gomez 2004. Permission Pending.
Mutations:
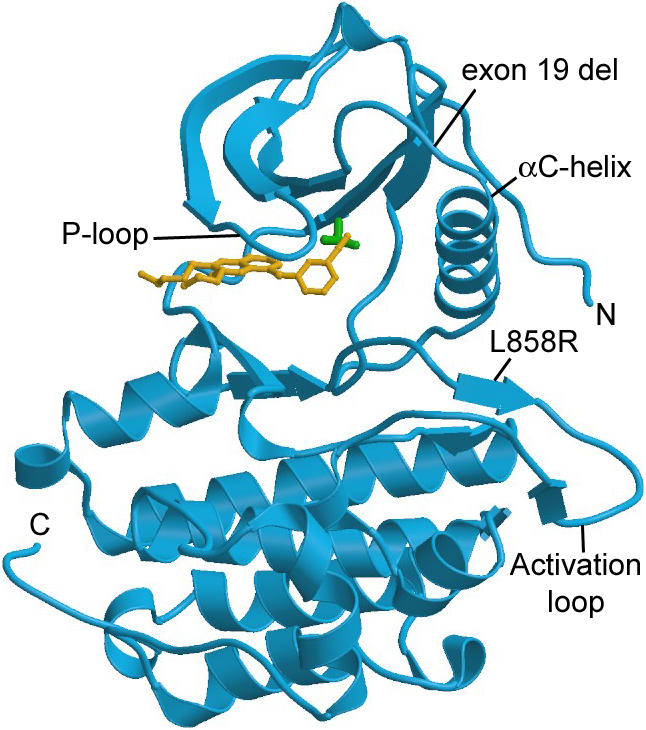
Figure 5: Erlotinib bound to EGFR tyrosine kinase, thus inactivating the receptor protein. Image from Clark. Permission Pending.
EGFR–tyrosine kinase is a heavily studied protein, because its mutations have been discovered to cause diseases like lung cancer and diabetes.In normal cells, the protein's function is highly regulated, however, in mutant cells it becomes unregulated (Clark, 2005). Since EGFR regulates cell division, and proliferation, a mutant EGFR can lead to the advancement of cancerous tumors by constantly remaining in the “on” position. In non-small cell lung cancers, 80% of the cancers have overexpressed EGFRs (Hongbin, 2005), and the cancer cells remain dependant on the mutated EGFR (Clark, 2005). The model study organism for studying EGFR mutants is the mouse. It is used as a candidate to test therapies and create tumors, because the mouse has a EGFR ortholog very similar to those of humans. Through the introduction of a mutant EGFR gene, the mouse can create mutagenic EGFRs (Hongbin, 2006). Through such a method, different therapies can be tested for their effectiveness at regulating the mutated EGFR, such as imatinib and erlotinib. Imatinib and erlotinib are tyrosine–kinase inhibitors (TKI), and they block the EGFR from becoming active (National Cancer Institute). The exact method of how the inhibitors work is unknown, but it is known that they work as inhibitors that compete with other ligands to bind with EGFR (National Cancer Institute, 2006). These treatments have been found to prolong the life of a patient with non-small cell lung cancer. Other treatments that have been created to interfere with EGFR are antibodies, like cetuximab, that bind to the EGFR to block the cell cascade that would normally occur if the EGFR binded with its ligand (Hongbin, 2006).
Additionally, there is a lot of research into the conferred resistance of EGFR- tyrosine kinases to its tyrosine kinase inhibitors (TKI). The resistance occurs as a result of a point mutation at thr 790, where met is substituted for thr (Kobayashi, 2005). Research has shown, that it is not simply the mutation that confers resistance, but it is the addition of the TKI before EGFR stimulation with EGF (Kobayashi, 2005). This is one hypothesis to explain the high rate of resistance to TKIs over time.
------------------------------------------------------------------------------------------------------------------------------------------------
Bogdan S, Klambt C. Epidermal Growth Factor Receptor Signaling. Current Biology 2001; 11: 292-295.
Clark J, Cools J, Gilliland D. EGFR Inhibition in Non-Small Cell Lung Cancer: Resistance, Once Again, Rears Its Ugly Head. PLoS Med 2005; 2(3): 195-197.
Erlotinib (Tarceva®) Extends Survival in Advanced Lung Cancer. National Cancer Institute. Avail from: http://www.cancer.gov/clinicaltrials/results/lung-and-erlotinib0604. 8/30/2006.
Ghandi T, Zhong J, Mathivanan S, Karthick L, Chandrika K. Analysis of the human protein interactome and comparison with yeast, worm, and fly interaction datasets. Nature Genetics 2006; 38: 289.
Gomez A, Volff J, Hornung U, Schartl M, Wellbrock C. Identification of a Second egfr Gene in Xiphophorus Uncovers an Expansion of the Epidermal Growth Factor Receptor Family in Fish. Molecular Biology and Evolution 2004; 21: 266-275.
Hongbin J, Danan L, Chen L, Shimamura T, Kobayashi S. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 2006; 9:485-495.
Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005; 352: 786–792.
LET-23 C. Elegans. Ensembl.org. Avail from: http://www.ensembl.org/Caenorhabditis_elegans/Gene/Summary?db=core;g=ZK1067.1.
Livneh E, Glazer L, Segal D, Schlessinger J, Shilo B. The Drosophila EGF receptor gene homolog: Conservation of both hormone binding and kinase domains. Cell 1985;40:599-607.
Shilo B, Scheiter E, Segal D, Ginsberg D, Glazer L. The Drosophila epidermal growth factor receptor homolog: structure, evolution, and possible functions. The Symposium of Fundamental Cancer Research 1986; 39: 87.
Schejter E, Shilo B. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell 1989; 56:1093.
Woodgett JR, editor. Protein Kinases. New York: Oxford, 1994. 177-202.
-------------------------------------------------------------------------------------------------------------------------------------
Please direct questions or comments to:my email