Human Prions
Introduction
Prions are proteins associated with a type of diseases called transmissible spongiform encephalopathies (TSE). TSE's are always fatal diseases that include the human diseases Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru, and the more well known bonvine disease mad cow. According to the "protein only" model, TSE's are caused by a conformational change of normal cellular prion proteins (PrP(C)) to the pathogenic form (PrP(Sc)) (Zahn et al. 2000).
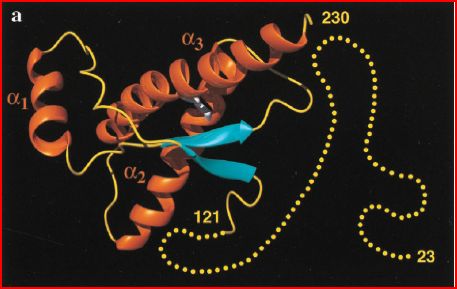
Figure 1. A cartoon of the three-dimensional structure of human prion protein. The alpha-helices are orange, the beta-strands blue, segments with nonregular secondary structure yellow, and the flexibly disordered tail of amino acids 23-124 is yellow dots. (Zahn et al. 2000)
Structure
The human prion protein five secondary structure features: 3 alpha helices and 2 beta strands. Alpha helix 1 includes amino acids 144 to 154, alpha helix 2 inclides amino acids 173 to 194, and alpha helix 3 is formed by amino acids 200-228. Beta strands 1 and 2 are composed by amino acids 128 to 131 and 161 to 164 respectively.
NMR analysis of the protein structure indicates several places that appear to have certain level of flexibility and resulting reduced precision of structure determination:
-In the final (C-terminal end) two turns of helices 2 and 3, the alpha helix appears to be in equilibrium with unfolded polypeptide forms
-The loop of amino acids 167 to 171, between beta-strand 2 and alpha helix 2, shows slow exchange between multiple polypeptide backbone configurations
-The protein contains a flexible N-terminus tail from amino acids 23 to 124
-There is a flexible C-terminal chain of amino acids 229 to 230
(Zahn et al. 2000)
Consequences of Variablity of Structure
Prions are proteins associated with a type of diseases called transmissible spongiform encephalopathies (TSE). TSE's are always fatal diseases that include the human diseases Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru, and the more well known bonvine disease mad cow. According to the "protein only" model, TSE's are caused by a conformational change of normal cellular prion proteins (PrP(C)) to the pathogenic form (PrP(Sc)) (Zahn et al. 2000).
Figure 1. A cartoon of the three-dimensional structure of human prion protein. The alpha-helices are orange, the beta-strands blue, segments with nonregular secondary structure yellow, and the flexibly disordered tail of amino acids 23-124 is yellow dots. (Zahn et al. 2000)
Structure
The human prion protein five secondary structure features: 3 alpha helices and 2 beta strands. Alpha helix 1 includes amino acids 144 to 154, alpha helix 2 inclides amino acids 173 to 194, and alpha helix 3 is formed by amino acids 200-228. Beta strands 1 and 2 are composed by amino acids 128 to 131 and 161 to 164 respectively.
NMR analysis of the protein structure indicates several places that appear to have certain level of flexibility and resulting reduced precision of structure determination:
-In the final (C-terminal end) two turns of helices 2 and 3, the alpha helix appears to be in equilibrium with unfolded polypeptide forms
-The loop of amino acids 167 to 171, between beta-strand 2 and alpha helix 2, shows slow exchange between multiple polypeptide backbone configurations
-The protein contains a flexible N-terminus tail from amino acids 23 to 124
-There is a flexible C-terminal chain of amino acids 229 to 230
(Zahn et al. 2000)
Consequences of Variablity of Structure
The
flexibility of the PrP(C) accounts for the fact that TSE's can have
spontaneous origins. As the regular cellular protein varies
between all the possible structures, there is the possiblity that one,
along with certain cellular conditions, could result in the necessary
conformational change from PrP(C) to PrP(Sc).
Works Cited
Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Garcia F, Billeter M, Calzolai L, Wider G, Wuthrich K. NMR solution structure of the human prion protein. PNAS 2000; 97 (1): 145-150. http://www.pnas.org/content/97/1/145.full.pdf+html. Accessed 2010 Feb 13.
Works Cited
Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Garcia F, Billeter M, Calzolai L, Wider G, Wuthrich K. NMR solution structure of the human prion protein. PNAS 2000; 97 (1): 145-150. http://www.pnas.org/content/97/1/145.full.pdf+html. Accessed 2010 Feb 13.
Please direct questions and comments to mamiller@davidson.edu