This web page was produced as an assignment for an undergraduate course at Davidson College.
The Sodium-Potassium Pump
FUNCTION
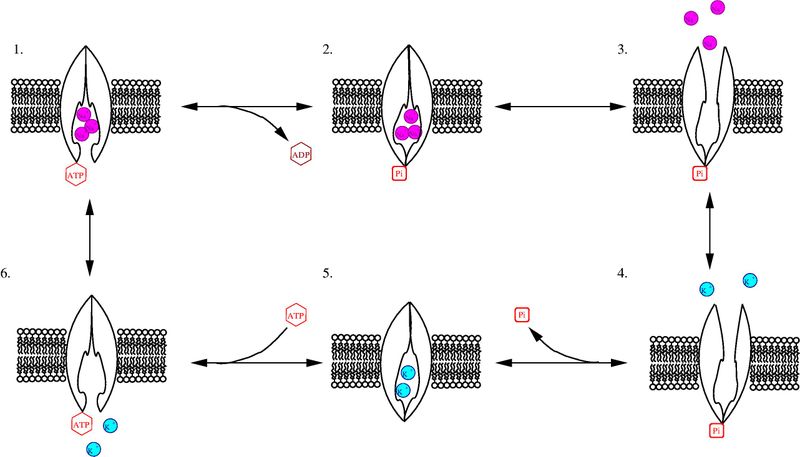
The sodium-potassium pump, also known as the Na,K-ATPase, a member of the P-type class of ATPases, is a critical protein found in the membranes of all animal cells. It functions in the active transport of sodium and potassium ions across the cell membrane against their concentration gradients (Morth et al., 2007). For each ATP the pump breaks down, two potassium ions are transported into the cell and three sodium ions out of the cell (Figure1).
Figure 1-
Purple Balls = Sodium Ions
Blue Balls =Potassium Ions
Image found on wikimedia commons and is open for reuse.
FIGURE 1 Walkthrough-
1. In conformation E1, three sodium ions bind to the pump from the cytoplasmic side of the phospholipid bilayer membrane.
2. ATP phosphorylates the enzyme, as indicated by the P attached to the cytoplasmic side of the pump, and the remaining ADP is released.
3. The conformation then changes to E2, and the three sodium ions are released into the extracellular space.
4. Two potassium ions bind to the pump from the extracellular side.
5. The pump is dephosphorylated and returns to conformation E1.
6. ATP binding occurs and the potassium is released into the cytoplasm.
The sodium-potassium pump creates an electrochemical gradient across cell membranes. The electrical gradient, created by the outflow of more positive sodium ions than the inflow of positive potassium ions, resulting in a relatively negatively charged cytoplasm,is used in neurons and muscles to create the action potentials responsible for nervous system function and muscular contraction. The chemical gradient, which is created by the higher concentration of sodium ions in the extracellular space as opposed to the cytoplasm, results in the tendency of sodium ions to flow down their concentration gradient and back into the cytoplasm through other transmembrane proteins. Cells use the chemical gradient to transport essential nutrients such as glucose and amino acids into the cell in a process called secondary active transport (Sadava, et al.,2006).
STRUCTURE
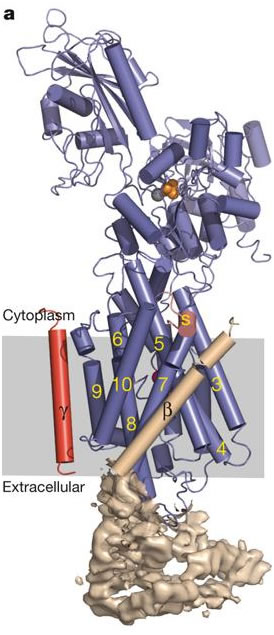
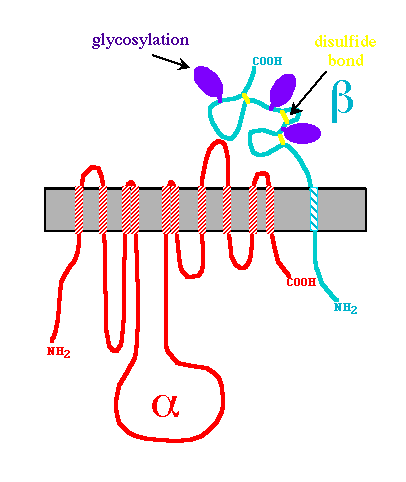
The Na, K-ATPase is composed primarily of one alpha subunit (approx. 1000 amino acids) and one beta subunit (approx.305 amino acids), which form the basic complex (Campbell, 2000). Another subunit, termed gamma, is sometimes associated with the alpha-beta complex and acts in tissue specific regulation. The alpha subunit is responsible for most of the enzyme's pumping function. It contains the specialized sequences of amino acids which bind to sodium, potassium, and ATP. The beta subunit is involved in the routing of the alpha-beta complex to the cell membrane, and it also functions to occlude, or to make inaccessible to either side of the membrane, potassium ions during conformation change (Morth, et al.,2007). The yellow numbers in figure 2 indicate the ten transmembrane segments of the alpha subunit. The beta subunit, unlike the alpha, contains only one transmembrane segment (the helix labeled with the Greek letter beta in figure 2). The alpha subunit dominates both the cytoplasmic and transmembrane regions of the enzyme, while the beta subunit is primarily on the extracellular side of the membrane. This arrangement makes sense given their specific functions. The alpha transmembrane amino acid sequences are important in ion binding and occlusion for transportation across the membrane, as well as complex formation with the beta subunit, which occurs between alpha-m7 and alpha-m10 (Morth, et al.,2007). Further bonding in the alpha-beta complex has been shown to take place between the extracellular segments of the alpha subunit with those of the beta subunit. It has been demonstrated that a 26 amino acid extracellular segment between the transmembrane segments alpha-m7 and alpha-m8 of the alpha subunit binds particularly well with the beta subunit. Another extracellular segment located between transmembrane segments alpha-m9 and alpha-m10 has also been implicated in alpha-beta complex formation (Lemas et al., 1993). The potential for association between the alpha amino acid segments with the beta subunit is depicted in cartoon form in figure 3. Note the locations in which both subunits are nearly touching or are in close proximity.
Figure 2- alpha, beta, and gamma subunits are shown as blue, wheat, and red respectively.
Permission pending from the Nature Publishing Group
Figure 3-
http://bio.davidson.edu/Courses/Molbio/26aa/alphabeta.html
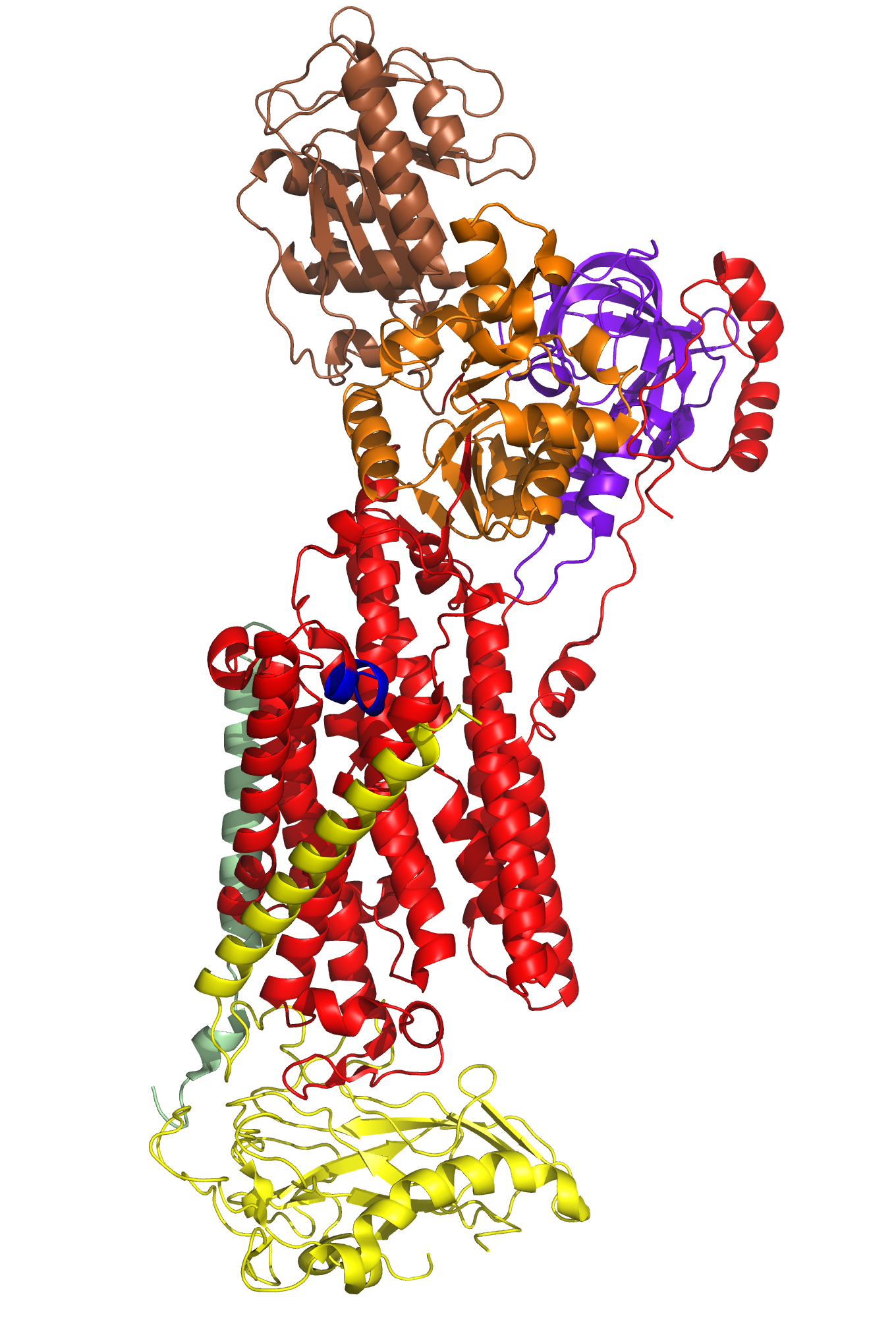
Particular portions and amino acid residues of each of the subunits are responsible for the Na,K-ATPase's specificity in function. For example, in addition to alpha-m7's role in the formation of the alpha-beta complex, this transmembrane helix is essential in the regulation of the potassium and sodium coordination sites located between residues of aplha-m4, alpha-m5, alpha-m6, and potentially aplha-m8 (Kim,2009/Morth et al., 2007). A particular amino acid residue of alpha-m7, Gly 855, is capable of distorting or "kinking" the shape of itself and the aforementioned transmembrane segments to enlarge the area of the potassium/sodium coordination sites, increasing their affinity for potassium ions(As seen in the animation at the bottom of this helpful page http://www.doe-mbi.ucla.edu/~yeates/153AH_2009_project/kim.html). The beta subunit, as mentioned earlier, is involved in stabilizing the coordination sites by occluding the ions closed within them. The majority of the beta subunit is appropriately placed on the extracellular side of the phospholipid membrane, allowing it to act as a lid for the potassium/sodium coordination sites. The C-Terminus (labeled blue in Figure 4) also plays an important role in regulating ion affinity of the potassium/sodium coordination sites. It inserts tyrosine residues to inhibit the Gly 855 kinking, reconfiguring the coordination site to favor sodium binding (Kim, 2009). The actuator, phosphorylation, and nucleotide domains of the alpha subunit are all located on the cytoplasmic side of the membrane. ATP binds to the nucleotide domain and eventually phosphorylates the phosphorylation domain. The actuator domain acts to couple this reaction to the manipulation of the transmembrane segments to open the occlusion site for sodium or potassium binding, depending on which state, E1 or E2, the protein is in.
Figure 4- Alpha subunit = Red - Beta Subunit = Yellow - Gamma subunit = Pale Green - Actuator Domain = Purpleblue - Nucleotide Domain = Brown - Phosphorylation Domain = Orange - CTerminus = Blue
http://www.doe-mbi.ucla.edu/~yeates/153AH_2009_project/kim.html
References-
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal Structure of the Sodium-Potassium Pump. Nature 2007; 450: 1043-1049
Sadava, Hellens, Orians, Purves, Hillis. Life: The Science of Biology. 8th ed. Gordonsville: Sinauer Associates; 2008.
Campbell AM. Sodium Pump Alpha and Beta Assembly. Wired [Online]. Avail from: http://bio.davidson.edu/Courses/Molbio/26aa/alphabeta.html [2000]
Lemas VM, Hamrick M, Takeyasu K, Fambrough DM. 26 Amino Acids of an Extracellular Domain of the Na,K-ATPase Alpha-Subunit are Sufficient for Assembly with the Na,K-ATPase Beta-Subunit. The Journal of Biological Chemistry 1994; 269(11): 8255-8259
Kim D. ATPases: Sodium/Potassium Pump (E2-2K*Pi Configuration). Wired [Online]. Avail from: http://www.doe-mbi.ucla.edu/~yeates/153AH_2009_project/kim.html [2009]
Go back Home
Go to the Molecular Web Page
Contact me at: ropalmer@davidson.edu