Heat Shock Protein Hsp90
Heat shock proteins are a family of proteins that act as chaperones for other proteins in prokaryotic or eukaryotic cells under stress, especially heat stress. The specific role they play is in helping other proteins fold correctly and in keeping nonnative polypeptides and incorrectly folded proteins from aggregating in the cytoplasm. Of these heat shock proteins, Hsp90 is the most abundant, and in cells in heat shock it is one of the most abundant proteins in a cell (Garnier et al 2002). Among the target proteins are v-Src, Weel, and c-Raf, along with others (Chadli et al 2000).

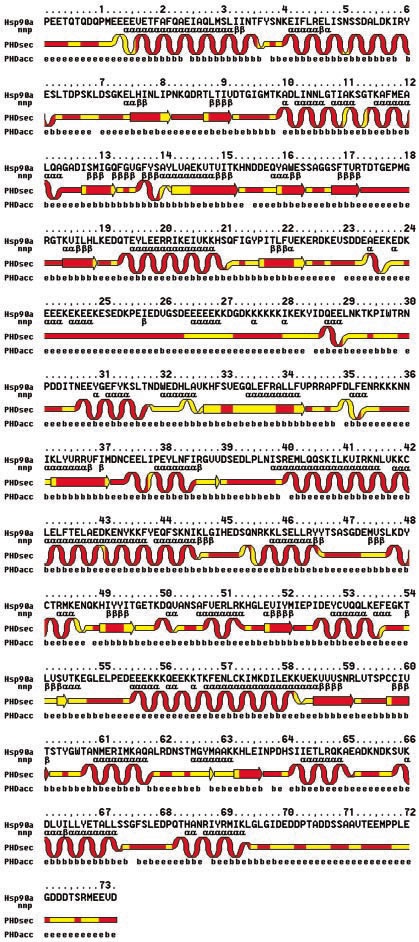
Figure 1 (Figure 1 from Garnier et al 2002). Primary Amino Acid sequence and computer predicted secondary structure of Hsp90 (Garnier et al 2002).
To understand the function of Hsp90, the structure of the protein and its folding must be analyzed. The general structure of the protein is that of a charged hinge region as the middle part of the molecule linking together the C-terminus and N-terminus. The N-terminal region is known to have a ATP-Mg/ADP-Mg binding site. This site provides a chaperone site to bind to the target protein with the expense of energy from ATP. This site can be inhibited from accepting ATP by geldanamycin (an antibiotic) and radicicol, preventing it from carrying out its function. At the C-terminal region, there is a second ATP binding site that also has a chaperone site to bind a target protein. Both ATP binding sites at the C-terminal and N-terminal regions are believed to be involved with the conformational change of the entire protein when target proteins are bound to it (Garnier et al 2002).
In order for Hsp90 to bind to target proteins and fulfill its function, it must first go through dimerization. In this dimerization, anti-parallel interactions between the 542-615 and 629-731 regions of the amino acid sequence at the C-terminal region allow the formation of the dimer (Garnier et al 2002). After dimerization, there are a few co-chaperones that must bind to the molecule in order for it to carry out its function. ATP must bind to the N-terminal ATP binding site, while several other proteins including p23, other heat shock proteins, and one of four separate tetraricopeptide repeat-containing proteins must also interact with the dimer. Where and how these proteins bind to Hsp90 are not clear at this time. However, this complex is still inactive when a protein called Hop is still bound to the ATP binding site at the C-terminal region. When this Hop protein is replaced with either an ATP molecule or an immunophilin, the the Hsp90 complex can perform its function (Chadli et al 2000).
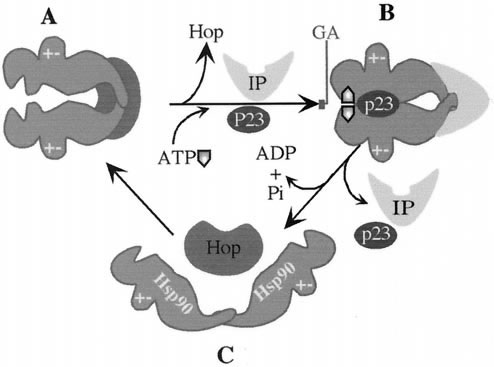
Figure 2 (Figure 7 from Chadli et al 2000). Pathway of dimerized Hsp90 complex. A is the functional Hsp90 complex when Hop is replaced by ATP and Immunophilin (IP), B is the Hsp90 complex bound to p23in its functional conformation, and C is the non-functional Hsp90 dimer with Hop bound to it (Chadli et al 2000).
For PDB structure, related literature, and other information, go to the Protein Data Bank Web Site
References
Chadli, Ahmed, Bouhouche, Ilham, Sullivan, William, Stensgard, Bridget, McMahon, Nancy, Catelli, Maria G., and David O. Toft. 2000. Dimerization and N-terminal domain proximity underlie the function of the molecular chaperone heat shock protein 90. PNAS, Vol. 97, no. 23, pp. 12524-12529.
Garnier, Cyrille, Lafitte, Daniel, Tsvetkov, Philipp O., Barbier, Pascale, Leclerc-Devin, Jocelyne, Millot, Jean-Mare, Briand, Claudette, Makarov, Alexander A., Catelli, Maria G., and Vincent Peyrot. 2002. Binding of ATP to Heat Shock Protein 90: Evidence fo an ATP-Binding Site in the C-Terminal Domain. The Journal of Biological Chemistry. Vol. 277, no. 14, pp. 12208-12214.
Contact: Shpurvis@davidson.edu