
A key characteristic of any immune system is the ability to recognize self from non-self. How can cytotoxic T lymphocytes distinguish between healthy cells and disease-infected cells? The answer lies within a group of membrane proteins - the major histocompatibility complex (MHC) superfamily. In particular, MHC class I proteins signal the immune system through antigen presentation. An MHC class I protein is anchored into the cell membrane and extends upright into the extracellular area. At the top of MHC class I is a small groove, which binds a specific peptide from a protein that has been digested within the cell. Tc cells have T cell receptors (TCRs) on their membranes that can bind to an MHC class I that is presenting a antigen. These TCRs can determine whether this peptide is representative of normal cell function or an implication that the cell has been infected.
In humans, the MHC proteins are called human leukocyte antigens (HLA). Hundreds of alleles coding for HLA proteins have been identified, but an individual can only express up to six different MHC class I molecules - three alleles from each parent (Kuby et al., 2007). However, each MHC molecule can bind many different peptides, allowing a wide range of possible antigens that can be presented in a person with six or less different MHC class I alleles. This variation in MHC alleles among individuals is the culprit of graft rejection. If a patient receives a graft from someone with different MHCs, the patient's immune system will recognize the foreign MHCs and destroy the donor tissue. The patient and the donor must be "histocompatible" for a successful graft (Goodsell, 2005).
MHC class I proteins are present on the membrane of all nucleated cells (thus, not on red blood cells). Naturally, the structure of MHC is crucial for proper function, and a mutation in a gene coding for an MHC can inhibit its ability to bind and present antigenic peptides. The next section examines the general structure of an MHC class I protein.
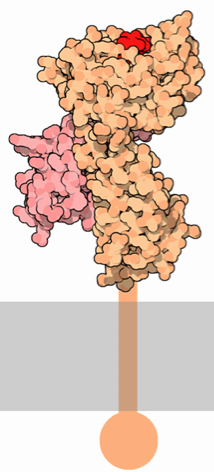
MHC class I proteins consist of two polypeptide subunits: an α chain (the "heavy" chain) and a β2-microglobulin molecule (Figure 1). The α chain is where the most variation is present among different MHC class I proteins. The α chain is organized into three extracellular α domains (α1, α2, and α3), a transmembrane region, and a cytoplasmic carboxyl tail. Each extracellular α domain is approximately 90 amino acids long, while the transmembrane region is 24 amino acids and the intracellular tail is 35 amino acids (Geier et al., 1986). The α1 and α2 domains fold with each other to form a deep groove, which is the peptide-binding cleft. Crystallographic analysis shows that the α1 and α2 domains point away from the cellular membrane in order for optimal exposure of the peptide-binding cleft to T cells (Celia et al., 1999). This antigen binding cleft can bind peptides 8 to 10 amino acids long. The α3 domain of the alpha chain associates with the β2-microglobulin, and the transmembrane region extends downward from the α3 domain into the cytoplasm of the cell, ending in a short carboxyl tail.

|

|
Figure 1. Representations of MHC class I. LEFT: The α chain (orange) spans the cellular membrane and extends extracellularly. The β2-microglobulin chain (pink) binds to the α chain and is critical for the function and stability of MHC class I. The small red polypeptide represents an antigen being presented by MHC. RIGHT: A simplified depiction of the MHC class I subunits, showing the membrane spanning domain, the three domains of the α subunit (purple) and the β2-microglobulin chain (pink). No antigen is bound to the cleft, showing that the groove is open-ended on both sides. Also note that this illustration does not depict the intracellular carboxyl tail. These images were obtained from Wikimedia Commons.
The β2-microglobulin chain is composed of β pleated sheets, and it interacts with all three domains of the α chain to provide molecular stability and appropriate affinity for specific peptides within the binding groove. Unlike the α chain, β2-microglobulin does not have a transmembrane domain, yet it is a critical portion of the MHC class I protein. Mutations in the α1 or α3 domains severely inhibit the binding of the heavy chain to β2-microglobulin. Particularly, the binding of both an antigen and β2-microglobulin to the α1 domain is essential for the proper folding of the entire MHC class I molecule (Duprat et al., 2006). In vitro studies have shown that there is no detectable expression of MHC class I on the surface of the cell in the absence of β2-microglobulin (Hill et al., 2003).
The purpose of MHC class I molecules is to alert cytotoxic T cells about intracellular activities. They accomplish this by binding small peptides and presenting them extracellularly. These peptides are fragments of intracellular proteins that have been produced in the cell and then digested with proteases. After digestion, the peptides are transported to the endoplasmic reticulum, where they can associate with MHC class I molecules. The entire MHC class I-peptide complex is transported to the surface of the cell, where the α chain of the MHC becomes anchored into the membrane and the peptide is bound to MHC extracellularly (Altman and Trowsdale, 1989).
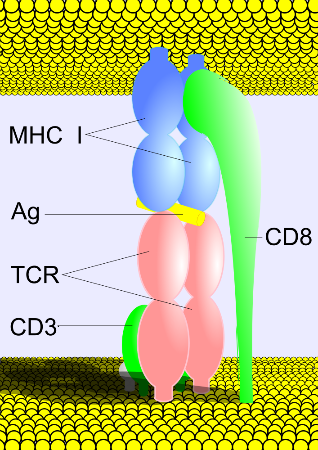
Cytotoxic T cells have TCRs specific for MHC class I, but what if the TCR accidentally binds the wrong cell membrane protein and doesn't recognize the antigen? There is a second binding that must occur in order to prevent the immune system from accidentally attacking healthy cells. TCRs have another surface protein called a co-receptor. Specifically, cytotoxic T cells have CD8 as a co-receptor. CD8 binds to a highly conserved region on the MHC class I molecule, and only with signals from both the TCR and CD8 can the cytotoxic T cell attack the infected cell (Figure 2).
Figure 2. A depiction of the binding ofthe TCR to MHC class I. The cytotoxic T cell (bottom) possesses a TCR (pink) with a CD8 co-receptor. CD3 is a cluster of differentiation associated with TCRs. The TCR is bound to the antigen-presenting MHC class I, and the CD8 is bound to the conserved region on MHC class I. This image was obtained from Wikimedia Commons.
Altmann DM and J Trowsdale. 1989. Major histocompatibility complex structure and function. Current Opinion in Immunology 2: 93-98.
Celia H, E Wilson-Kubalek, RA Milligan, and L Teyton. 1999. Structure and function of a membrane-bound murine MHC class I molecule. Proc. Natl. Acad. Sci. 96: 5634-5639.
Duprat E, MP Lefranc, and O Gascuel. 2006. A simple method to predict protein-binding from aligned sequences - application to MHC superfamily and β2-microglobulin. Bioinformatics 22: 453-459.
Geier SS, RA Zeff, DM McGovern, TV Rajan, and SG Nathenson. 1986. An approach to the study of structure-function relationships of MHC class I molecules: Isolation and serologic characterization of H2-Kb somatic cell variants. J. Immunol. 137: 1239-1243.
Goodsell DS. 2005. Major Histocompatibility Complex. RCSB Protein Data Bank. http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb62_1.html 14 February 2010.
Hill DM, T Kasliwal, E Schwarz, AM Hebert, T Chen, E Gubina, L Zhang, and S Kozlowski. 2003. A dominant negative mutant β2-microglobulin blocks the extracellular folding of a major histocompatibility complex class I heavy chain. The Journal of Biological Chemistry 278: 5630-5638.
Kuby J. 2007. The major histocompatibility complex and antigen presentation. In: Immunology (TJ Kindt, RA Goldsby, and BA Osborne). WH Freeman and Company, 189-222.