FOXP2: The Human Language Gene |
This web page was produced as an assignment for an undergraduate course at Davidson College.
Primary Research Article
Background
Mutations within the gene for the Forkhead-box transcription factor FoxP2 cause a monogenic speech and language disorder. Heterozygous FOXP2 mutations cause developmental verbal dyspraxia, characterized by difficulties mastering complex mouth movements, expressive language, and basic sentence processing; while other cognitive processes are normal (Fisher and Scharff 2009). However, with adequate training, affected children can become competent speakers. This suggests that FOXP2 has a broad role in the formation of words and language (Gibson and Gruen 2008). As a transcription factor, the downstream pathways FoxP2 regulates could also have broader relevance for common language impairments.
To date, genomic screening of FOXP2 targets by chromatin-immunoprecipitation uncovered the gene CNTNAP2, a member of the neuronal transmembrane proteins involved in cell adhesion. Studies of CNTNAP2 and other genes containing FOXP2-binding motifs found that the FoxP2 protein usually represses transcription of target genes. (Vernes et al 2008) Now the challenge has been to define transcriptional targets and relate them to neural function and the complex essence of language: grammar, abstraction, meaning, and thoughts (Fisher and Scharff 2009).
Although no animal model entirely mirrors the levels of complexity of human language, investigations of FOXP2 in other species have uncovered valuable information. A study of vocal learning in songbirds found that FoxP2 knockdown zebra finches imitated tutor songs incompletely, omitting some notes and varying syllable production – similar to inaccurate word rendition and variable pronunciation in humans with verbal dyspraxia. (Haesler et al 2007) Moreover, the same area of the brain, the striatum of the basal ganglia, is affected in dyspraxia patients and the knockdowns. Homozygous mutation or knockout of FOXP2 in mouse models causes severely compromised motor skills and death 3-4 weeks after birth (Fisher and Scharff 2009).
In Enard et al’s 2009 Cell article, researchers address the functional consequences of evolutionary differences in the human FOXP2. Two amino acid substitutions (T303N, N325S) separate human FOXP2 from chimpanzees, and only one conservative substitution separates chimps from mice. Enard and colleagues successfully constructed a mouse strain with the human T303N and N325S substitutions in FOXP2.
Results of Enard et. al 2009 (See a video summary of this article online.)
Mice with two alleles for "humanized" FOXP2 (FOXP2 hum/hum) were compared with mice with one wild-type FOXP2 allele (FOXP2 wt/ko) in order to simulate the functional differences between normal human FOXP2 and people with language disorders due to a nonfunctional FOXP2 allele. Mice were subjected to numerous behavioural tests, and results were normalized to homozygous wild-type (FOXP2wt/wt) mice. The figure below shows behaviours that were significantly different between strains. Overall, humanized FOXP2 mice showed less exploratory behaviour than wild-type and FOXP2wt/ko mice. However, humanized FOXP2 mice participated in significantly more group contact than either other strain. Humanized FOXP2 had no notable effects on other tested behaviour or any other organ.
Dopamine levels were tested throughout various regions of the brain to study the effect of FOXP2 on this organ. In humanized FOXP2 compared to wild-type and FOXP2wt/ko, there was a reduction of dopamine levels in all regions of the brain, and no notable effects on any other neurotransmitters. Enard suggests that the dopamine levels are causally linked to the observed behaviour differences particular between FOXP2hum/hum and FOXP2wt/ko. The increase in dopamine tends to increase exploratory behaviour and vice versa.
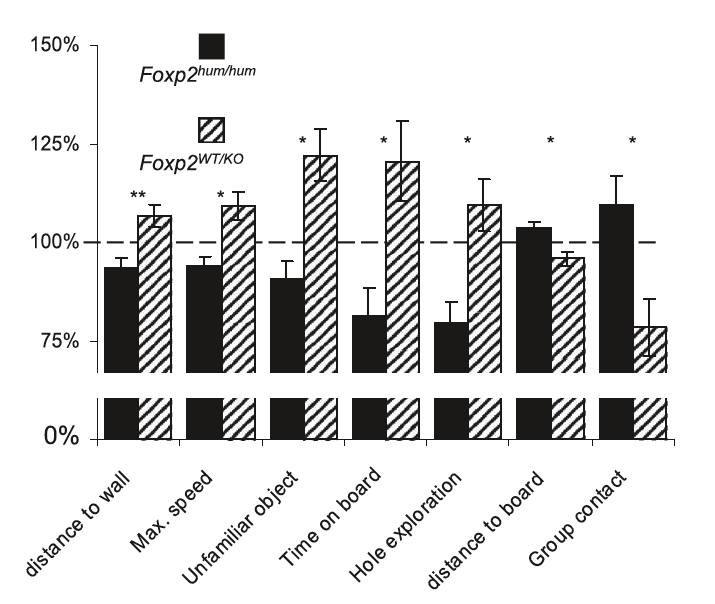
Figure 2. Extracted from Enard et al. 2009.
FoxP2, however, is not expressed in dopaminergic neurons. It is expressed in medium spiny neurons - the major targets of dopaminergic neurons - that make up 90% of neurons in the striatum. As previously noted, the striatum is known to be the affected area in patients with developmental verbal dyspraxia and in FOXP2 knockdown songbirds. This staining of the cerebellum (right) shows FoxP2 (red) and calbindin (green) in Purkinje cells and medium spiny neurons, which have roles in controlling body movements. |
Figure S6-E. Extracted from Enard et. al 2009 Supplemental Material. |
Narrowing the investigation to the medium spiny neurons, neural cells from FOXP2hum/hum and FOXP2wt/wt mice were grown in vitro. They found that neurons with humanized FOXP2 grew longer neurites (Figure 4A below). In vivo, they found that dendrites were on average 22% longer than wild-type mice (Figure 4B). FOXP2wt/ko mice that are meant to model verbal dyspraxia had shorter dendrites than wild-type, but this difference was not statistically significant.
These FOXP2hum/hum neurons were further found to have twice as strong of a long-term synaptic depression after stimulation compared to FOXP2wt/wt. These data support the conclusion that humanized FOXP2 increases the synaptic plasticity in medium spiny neurons, and a nonfunctional FOXP2 allele has the opposite effect.

Figure 4. Extracted from Enard et. al. 2009.
| As a final test, the influence on vocalization in humanized FOXP2 mice was measured (Figure 6, right). Frequency measurements (kHz) were taken for FOXP2hum/hum mouse pups and FOXP2wt/wt pups at the points labeled in Figure 6A. Overall, the humanized FOXP2 mouse pups made lower-frequency sounds than wild-type pups when put in isolation. Although this finding is very interesting and reproducible, Enard et al. remark "it is important to note that this influence is subtle and within the range of normal variation among mice." |
Figure 6. Extracted from Enard et. al. 2009. |
Conclusions and Discussion
In conclusion, of particular interest in this article were the findings that exploratory behaviour, dopamine levels, and synaptic plasticity were all affected in opposite directions in FOXP2hum/hum and FOXP2wt/ko mice. Enard et al. argues that some of these effects could model aspects relevant for speech and language in humans.
The primary research article is a lot more detailed with specialized language that readers outside of this field of biology could not be expected to follow. However, the findings of the research are explained in a more logical progression than in the popular press article, which instead just provides the "exciting" findings and implications of the research article.
© Copyright 2010 Department of Biology, Davidson College, Davidson, NC 28035