Structure and Function
Mutations of Jak3 and Related Disorders
Jak3 Related Treatments for Immunodeficiency
Disorders
Alterations of Jak3 Signaling
References
Jak3 is a member of the Janus kinase family of proteins which is comprised of Jak1, Jak2, Jak3, and Tyk2. These proteins bind to cytokine receptors and play an essential role in cytokine signaling (Chen and others 1997). Of these, Jak3 is unique in its expression and association. Jak3 is highly expressed in hematopoetic cells (Witthuhn and others 1994). Furthermore, it binds specifically to the common gamma chain (gc), which is a shared component of the Interleukin 2 (IL-2), IL-4, IL-7, IL-9, IL-15 receptors . These receptors are known to cause proliferation and differentiation of lymphocytes (Miyazaki and others 1994). The Jak3-gc interaction has proven to be of great interest due to its role in the causation of several different types immunodeficiency disorders (Candotti and others 1997, Russell and others 1994, Thomis and Berg 1997). The intense research in this area has important implications including treatment of Severe Combined Immunodeficiency (SCID) and production of novel immunosuppressant drugs.
Structure and
Function (No PDB file currently available)
Jak3 functions as a key element in the signaling of cytokines.
Jak3 is known to associate with the gc chain
of receptors for IL-2, IL-4, IL-7, IL-9, and IL-15, and is activated by
these cytokines. In order to elucidate the role of Jak3 in the cytokine
signaling pathway, the IL-2 pathway will be used as an example. IL-2
causes the heterodimerization or oligomerization of IL-2R chains a,
b,
and gc. Of relevance to Jak3 is the dimerization
of the IL-2Rb and gc
chains to both of which Jak3 binds. When dimerized these chains bind
Jak3 and another member of the Janus kinase family, Jak1 (See Fig. 1).
After binding Jak1 and Jak3 are activated presumably through auto- and
trans-phosphorylation of the associated Jaks (Zhu and others 1998).
These activated Jaks induce rapid tyrosine phosphorylation of signal transducers
and activators of transcription 5 (STAT5) which then dimerizes through
reciprocal phosphotyrosine-SH2 domain interactions. Upon dimerization
STAT5 translocates into the nucleus and regulates transcription of target
genes (Liu and others 1997).

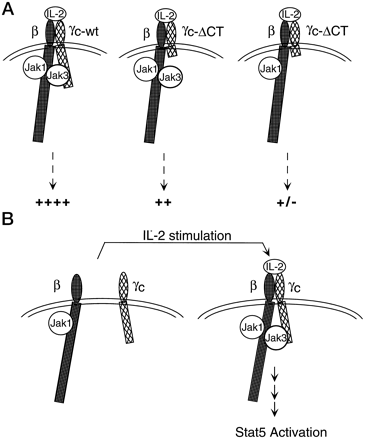
Fig. 1: Schematic model demonstrating
binding of Jak3 to both IL-2Rb and gc.
A) (in order from left) Wildtype gc asscociates with
Jak3 and is activated fully. Truncated gc
cannot bind Jak3 and is partially
activated. Truncated gc
cannot bind to Jak3 and no functional Jak3 is present, thus there is no
activation
B) The formation of the IL-2Rb and
gc
heterodimer, stabilization of the complex by Jak3 and ensuing to signal
cascade.
(Zhu and others 1998) Source:
http://www.jbc.org/cgi/content/full/273/17/10719/F9
Permission requested, Figure will be removed if denied
Jak proteins are between 1100 and 1200 amino acids and are divided
into seven structural domains known as Jak homology (JH) domains (see Fig.
2). Liu (1997) demonstrates that the carboxyl JH1 region of the protein
contains the activation loop, a region that contains the tyrosine kinase
catalytic domain. JH1 has also been shown to be regulatory region.
Multiple sites of autophosphorylation have been identified. Furthermore
two tyrosine residues, Y980 and Y981, positively and negatively regulate
the Jak3 kinase activity, respectively (Zhu and others 1998). The
JH7-6 domains have also been shown to be important to the function of Jak3.
The amino terminal JH7-6 domains (aa 1-192) are the minimal region necessary
for gc association with Jak3. A model
has been proposed which explains binding. The JH7-6 domains of Jaks
contain loosely conserved region as well as highly variable regions.
The loosely conserved regions can bind to conserved membrane proximal regions
termed Box1 and Box2 in cytokine receptors. The highly variable regions
thus determine ligand specificity, gc as in
the case of Jak3 (Chen and others 1997).

Fig. 2: The organization of
the Jak homology (JH) domains of the Jak3 protein (Notarangelo and Vihinen
1999).
Source:
http://www.uta.fi/imt/bioinfo/graphics/JAK3dom.gif
See
Copywrite Notice: http://www.uta.fi/imt/bioinfo/JAK3base/copyr.html
Another important function of Jak3 is its role in negative selection. Jak3-deficient mice when seeded with precursor cells were found to possess autoreactive T cells in the thymus and in the periphery. However, no autoimmune disease developed, indicating that the autoreactive T cells were anergic. The exact mechanism of this phenomenon is unknown, but several have been proposed. A Jak3-mediated growth signal may cause the deletion of autoreactive thymocytes in conjunction with the signals from the TCR. Other evidence supports the possibility that Jak3 is directly involved in T cell activation, but no conclusive evidence exists (Saijo and others 1997).
Mutations of Jak3 and Related Disorders
The cause for the immense amount of research on Jak3 is its role in
several forms of immunodeficiency diseases. Approximately 50% of
all SCID cases are caused by mutations in the gc
chain resulting in an X-linked SCID phenotype. Jak3 mutations result
in an autosomal recessive SCID that accounts for about 10% of all cases.
The phenotypes of these two forms of SCID are indistinguishable as are
the defects in the signaling pathways. An interesting note is that
while humans with these mutations have B+ T- NK- SCID, mice with the same
condition suffer from profound B lymphopenia as well (Brown and others
1999). The Jak3 -/- SCID mouse was found to have a diminished thymus
and a lack of peripheral lymph nodes and Peyer’s patches. However,
the thymocyte development and splenic T cells appeared normal. The
T cell defects occurred in the proliferative response and in secretion
of IL-2 in the presence of mitogen. Jak3 -/- mice also had B cell
development arrested at the pre-B stage, presumably indicative of a defect
in the IL-7 signaling pathway (Thomis and others 1995). Thomis and
Berg (1997) further elucidate the defect in the T cells of Jak3 -/- mice.
A transgenic reconstitution of the T cell function was performed with one
transgenic line expressing Jak3 in the thymus and in the periphery and
another transgenic line just expressing Jak3 in the thymus. Peripheral
T cells in Jak3 -/- mice were found to resemble activated or memory T cells
as were the peripheral T cells from the transgenic line expressing Jak3
only in the thymus. Conversely, the transgenic line that expressed
Jak3 ubiquitously was found to have normal peripheral T cells. Thus,
the cause of the phenotypic and functional defects in peripheral Jak3 -/-
T cells were found to be a result of acquired defects in the periphery
and not due to aberrant development within the thymus (Thomis and Berg
1997).
The membrane proximal regions of cytokine receptors contain the Box1
and Box2 motif that is essential to Jak activation and signal transduction.
Jaks associate directly with these regions; moreover, a single point mutation
has been shown to be able to disrupt this association sufficiently to cause
SCID when the mutation occurs in the JH7 domain of Jak3. Point mutations
gc
can also result in SCID as well as XCID, an immunodeficiency disorder that
still retains some function. A chimeric kinase was created using
the JH7-6 domains of Jak3 with JH5-1 of Jak1. The resulting kinase
was able to functionally substitute for Jak3 in the IL-2 receptor, indicating
that the N-terminal JH7-6 domains determine binding with the cognate receptor
in Jaks (Cacalano and others 1999).
Jak3 Related Treatments for
Immunodeficiency Disorders
As a result of the intense research into the Jak3 -/- SCID, some novel
approaches to treatment have been developed. The typical treatment
of SCID is irradiation and bone marrow transplant (BMT). This is
a cure if successful, but BMT is potentially dangerous and requires an
HLA-matching donor, of which there are few (Bunting and others 1999).
Brown (1998) demonstrates that the IL-3 pathway reconstitutes early lymphoid
proliferation and function in Jak3 -/- mice. This alternative pathway
could provide a means by which XSCID and Jak3 -/- SCID patients could receive
treatment. Gene therapy is also becoming a viable approach.
Retroviral-mediated gene transfer of murine Jak3 into the bone marrow of
a Jak3 -/- mouse has been shown to reconstitute the T and B lymphocytes
to sufficient levels to withstand a viral infection that had 100% mortality
in Jak3 -/- mice without the gene therapy. This result has clear
implications in human SCID and is entering human preclinical experimentation
(Bunting and others 1999).
Alterations of Jak3 Signaling
No drugs are currently known to affect the Jak3 protein, although such
a drug could have tremendous potential as a powerful immunosuppressant.
However, staphylococcal enterotoxins disrupt the Jak/Stat pathway sufficiently
to render T cells unresponsive to IL-2, leading to anergy and apoptosis
(Nielsen and others 1995). Conversely, the Epstein-Barr virus latent
membrane protein 1 (LMP1) inhibits apoptosis resulting in immortalized
B cells. The purpose of this response is not entirely clear, but
likely has to do with cell transformation and induction of growth in the
context of an EBV infection (Gires and others 1999).
Bunting KD, Flynn KJ, Riberdy JM, Doherty PC, Sorrentino BP. 1999.
Virus-specific immunity after gene therapy
in a murine model of severe combined immunodeficiency.
Proceedings of the National Academy of Science
96:232-237. <http://www.pnas.org/cgi/content/full/96/1/232>
Accessed 2000 Feb 29.
Brown MP, Nosaka T, Tripp RA, Brooks J, van Deursen JMA, Brenner MK,
Doherty PC, Ihle JN. 1998.
Reconstitution of early lymphoid proliferation
and immune function in Jak3-deficient Mice by interlekin-3.
Blood 94(6):1906-1914.
Cacalano NA, Migone TS, Bazan F, Hanson EP, Chen M, Candotti F, O’Shea
JJ, Johnston JA. 1999. Autosomal
SCID caused by a point mutation in the N-terminus
of Jak3: mapping of the Jak3-receptor domain. EMBO
Journal 18(6):1549-1558.
Candotti F, Oakes SA, Johnston JA, Gilliani S, Schumacher RF, Mella
P, Fiorini M, Ugazio AG, Badolato R, Bozzi
F, Macchi P, Strina D, Vezzoni R, Blaese RM,
O’Shea JJ, Villa A. 1997. Structural and functional basis for
JAK3-deficient severe combined immunodeficiency.
Blood 90(10):3996-4003.
<http://www.bloodjournal.org/cgi/content/full/90/10/3996>
Accessed 2000 Feb 28.
Chen M, Cheng A, Chen Y, Hymel A, Hanson EP, Kimmel L, Minami Y, Taniguchi
T, Changelian PS, O’Shea JJ.
1997. The amino terminus of Jak3 is necessary
and sufficient for binding to the common g chain and confers the
ability to transmit interleukin 2-mediated
signals. Proceedings of the National Academy of Science
94:6910-6915. <http://www.pnas.org/cgi/content/full/94/13/6910>
Accessed 2000 Feb 27.
Gires O, Kohlhuber F, Kilger E, Baumann M, Kieser A, Kaiser C, Zeidler
R, Scheffer B, Ueffing M,
Hammerschmidt W. 1999. Latent
membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates
STAT proteins. EMBO Journal 18(11):3064-3073.
Liu KD, Gaffen SL, Goldsmith MA, Greene WC. 1997. Janus
kinases in interleukin-2-mediated signaling: Jak1
and Jak3 are differentially regulated by tyrosine
phosphorylation. Current Biology 7:817-826.
Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z, Oishi
I, Silvennoinen O, Witthuhn BA, Ihle JN,
Taniguchi T. 1994. Functional
activation of Jak1 and Jak3 by selective association with IL-2 receptor
subunits.
Science 266:1045-1047.
Nielsen M, Svejgaard A, Ropke C, Nordahl M, Odum N. 1995.
Staphylococcal enterotoxins modulate interleukin 2
receptor expression and ligand-induced tyrosine
phosphorylation of the Janus protein-tyrosine kinase 3 (Jak3)
and signal transducers and activators of transcription
(Stat proteins). Proceedings of the National Academy of
Science 92:10995-10999. <http://www.pnas.org/cgi/reprint/92/24/10995>
Accessed 2000 Feb 29.
Notarangelo LD, Vihinen M. 1999 Mar 31. JAK3base: Mutation
registry for autosomal recessive severe combined
JAK3 deficiency. <http://www.uta.fi/imt/bioinfo/JAK3base>
Access 2000 Mar 02.
Russell SM, Johnston JA, Noguchi M, Kawamurra M, Bacon CM, Friedmann
M, Berg M, McVicar DW, Witthuhn
BA, Silvennoinen O, Goldmann AS, Schmalstieg
FC, Ihle JN, O’Shea JJ, Leonard WJ. 1994. Interaction of
IL-2Rb and gc
C\chains with Jak1 and Jak3: Implication for XSCID and XCID. Science
270:1042-1045.
Saijo K, Park SY, Ishida Y, Arase H, Saito T. 1997. Crucial
role of Jak3 in negative selection of self-reactive
T cells. J Experimental Medicine 185:
351-356. <http://www.jem.org/cgi/content/full/185/2/351>
Accessed 2000 Feb 28.
Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. 1995.
Defects in B lymphocyte maturation and
T lymphocyte activation in mice lacking Jak3.
Science 270:794-797.
Thomis DC, Berg LJ. 1997. Peripheral expression of Jak3
is required to maintain T lymphocyte function.
J Experimental Medicine 185:197-206. <http://www.jem.org/cgi/content/full/185/2/197>
Accessed 2000
Feb 26.
Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu, ET, Ihle
JN. 1994. Involvement of the JAK-3 Janus
Kinase in signaling by interleukins 2 and
4 in lymphoid and myeloid cells. Nature 370:153-157.
Zhu M, Berry JA, Russell SM, Leonard WJ. 1998. Delineation of
the regions of Interleukin-2 (IL-2) Receptor b
chain important for association of Jak1 and
Jak3. J. Biology Chemistry 273(17):10719-10725.
<http://www.jbc.org/cgi/content/full/273/17/10719>
Accessed 2000 Feb 26.
Return To Immunology Home
Page of
Charles Haines
Return
To Immunology Main Page
Send comments, questions, and suggestions to: chhaines@davidson.edu