This web page was produced as an assignment for an undergraduate course at Davidson College.
Jmol image of DAF (CD55) from Protein Data bank.
What is DAF?
Decay-accelerating factor (DAF), or CD55, is a 70kDa protein classified as a memeber of the family of complement activation regulators. This family also includes complement receptor 1 (CR1), CR2, and membrane cofactor protein (MCP) (Hasan et al., 2002). This protein is present on the surface of all blood and tissue cells. It is also present in a soluble form that is present in bodily fluids (Medof et al., 1987). It plays a vital role in protecting healthy (or oncogenic) cells from the damaging effects of the complement cascade. It is upregulated by tumor necrosis factor-α and endothelial growth factor which implicates its roles in inflammatory responses and angiogenesis (Mason et al., 2001; Ahmed et al., 2003). However, DAF has been found to provide a binding location for human enteroviruses through lipid rafts in the cell membrane (Stuart et al., 2002), as well as provide a binding site for certain E. coli adhesins (Hasan et al., 2002). This protein has also been implicated in many bodily functions including protection of the placenta and fetus during development (Girardi et al., 2006) and mediation of inflammed atherosclerotic plaques (Mason et al., 2002), but also protecting tumor cells as they grow and damage surrounding tissue (Fishelson et al., 2003). To understand the function and importance of this protein, it must be described within the context of the complement system.
Regulation of the Complement System (Overview)
The complement system is part of the innate immune system and is made up of plasma proteins that can activate each other in order to produce peptides that defend the body against infection. These complement peptides work to recruit inflammatory cells, opsonize pathogens, and/or kill them. There are three complement pathways that can be initiated, all of which lead to the formation of a C3 convertase. C3 convertases cleave the complement protein C3 into C3a and C3b peptides. C3b binds to cells and pathogens and initiates the process by which the cell is engulfed or lysed (Janeway et al., 2005). Of the three complement pathways, the classical and alternative pathways are implicated in DAF complement regulation. The classical pathway is controlled by membrane-bound DAF by its ability to bind the C3 convertase C4b2a. The alternative pathway is controlled by DAF binding to C3 convertase, C3bBb (Kuttner-Kondo et al., 2001). By binding and dissociating the C3 convertases in both paths, C3b peptides can no longer be produced to bind to the surface of the cells. DAF can also bind and dissociate C5 convertases on the surface of cells, which mediate the formational of membrane-attack complexes (Hasan et al., 2002). Therefore, the cells protected by DAF are not engulfed by phagocytic cells or lysed by membrane attack complexes.
DAF (CD55) Structure and Function
DAF is made up of four short consensus repeat (SCR) domains that take a linear rod-like form. X-ray diffraction of this protein has also revealed a serine/threonine/proline-rich region and a C-terminal glycosylphosphatidylinositol (GPI) anchor. The entire stalk length is 177 angstroms. SCR domains 2 and 3 contain residues that bind C3 convertase of the classical complement pathway. SCR domain 4 also contains residues that bind C3 convertase of the alternative pathway . DAF has been found to have low affinities for individual proteins C3b and Bb, but it has high affinity for both C3bB and C3bBb complexes. Experimentally, DAF only caused decay of the C3bBb complex. After dissociation of the convertase, the DAF-C3(5) convertase affinity is lost, and it dissociates (Lukacik et al., 2004). Another study also found that DAF binds the C3bBb complex with more affinity than to the individual protein parts. It binds molecules preferentially in the following order: Bb, C3b, and fB. Association of Bb subunit increases the avidity of the binding between DAf and C3bBb. The low affinity for the parts of the C3(5) convertases helps DAF to recycle and bind other convertases (Harris et al.2005).

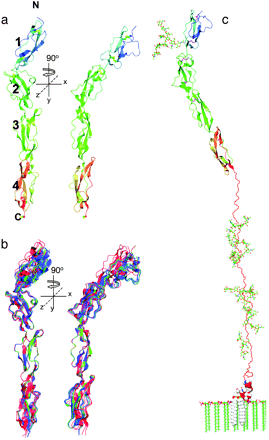
Figure 1. Structural models of DAF (CD55). (a) The structure of the four SCR domains with notations of the N terminus and C terminus. (b) Representation of three different crytalized forms overlaid to show the minimal variance found in SCR domains 2 and 3 between structural isolates. Form A is red, form B is green, and form C is blue. (c) This is a model of DAF shown with its GPI anchor in the cell membrane along with the N- and O-linked sugars (Lukacik et al., 2004). Copyright 2004, National Academy of Sciences.
The solution stucture analysis of an active fragment of DAF by Uhrinova et al. (2003) showed that there are four probable contact points between DAF and a classical pathway C3 convertase. Three of the contact points lie within the concave "front" surface on domains 2 and 3 of the structure. There is also a "hypervariable" loop in domain 2, amino acids 75-82, that accounts for structural variations found among DAF proteins (Uhrinova et al., 2003). Kuttner-Kondo et al. (2001) also found that the contact regions of DAF were found mainly on domains 2 and 3. Hydrophobic moieties along with a positively charged surface area on both domains accounted for the binding entities between DAF and C3 convertase of the classical pathway (Williams et al., 2003). The discovery of binding sites for alternative pathway C3 convertase and C5 convertase on domain four was later confirmed by Lukacik et al. (2004).
DAF deficiency
Gene Knockout Mouse models
Experiments using mouse models with DAF deficiency (DAF -/-) have helped characterize some of the phentypes and conditions associated with DAF deficiency. These conditions arise because of the inability of cells to break down immune complexes and convertases. Lin et al. (2002) found that DAF deficient mice develop autoimmune myasthenia gravis, an autoimmune neuromuscular transmission disorder that involves the loss of functional acetycholine receptors. In other studies by Lin et al. (2002), they found that DAF deficiency also resulted in autoantibody-induced glumerulonephritis and dextran sodium sulfate-induced inflammatory bowel disease. The DAF deficiency is also associated with the buildup of immune complexes within the blood. Mass deposition in kidney glumeruli podocytes initiates the classical complement pathway due to the tissue damage. This provides a mechanism for the development of glumerulonephritis in DAF deficient mice (Kim and Song, 2006).
Human Hematological Disorders
DAF deficiency in humans has been shown to play a part in the development of paroxysmal nocturnal hemoglobinuria. The lack of DAF results in intravascular hemolysis, increased platlet sensitivity, and elevated risk of thrombosis and stroke (Parker et al., 2005). The loss of DAF expression was attributed to somatic mutation of the PIG-A gene in hematopoietic stem cells (Miyata et al., 1994).
Vascular System Implications
DAF in also implicated in controlling complement-mediated hyperacute rejection associated with xenotransplantation organs. The endothelial cells of transplanted organs initiate complement cascades that result in destruction of these endothelial cells and coagulation of the vessels in a short period of time (Saadi and Platt, 1998). Transgenic pigs that overexpress DAF and other complement regulatory proteins (MCP and CD59) have been efficient at circumventing this problem and increasing the survival time after transplantation (Fecke et al., 2002).
Daf and Pregnancy
Complement activation regulation may be most important in the case of pregnancy where the embryonic cells and developing placenta must express complement regulatory proteins to prevent complement attack. Trophoblasts form the lining of chorionic villi in the placenta that separates the mother and fetus. A study of the trophoblasts in the placenta revealed that DAF (to a smaller extent) and other complement regulators are expressed by these cells and play an important role in preventing complement activation there. Lack of vital complement regulatory proteins, like DAF, is thought to be responsible for some cases of spontaneous abortion (Morgan and Holmes, 2000; Girardi et al., 2006).
DAF and Cancer
DAF and other complement regulatory proteins are upregulated in some types of cancer involving the reproductive tract cells (endometrial, prostate, ovarian cancer), blood cells (leukemia), and skin cells (melanoma). These proteins protect the cancerous cells as they grow and initiate complement cascades. They also pose a problem for anti-cancer antibody based therapies being developed. Complement regulator blocker molecules are being investigated that can selectively block DAF and other complement regulators on cancerous cells while leaving healthy cells unaffected (Fishelson et al., 2003).
Therapeutic Implications
Statin medications (Atorvastatin, Simvastatin, and Mevastatin) prescribed to hyperlipidemic patients have been shown to increase the expression of DAF on endothelial cells. The benfits heightened DAF expression can be seen in patients with normal-range LDLcholesterol as well. This increase of DAF on endothelial cells helps to mediate inflammatory responses in atherosclerotic plaques associated with high cholesterol. The upregulation of DAF also presents a mechanism for the general protection of all endothelial cells and prevention of inflammatory vascular diseases that involve complement activation (Mason et al., 2002).
Experiments have also been done that involve fusing rat DAF to Fc fusion proteins to produce DAF-Ig molecules. The molecules produced are antibody-like except they have DAF proteins where Fab fragments should be. These proteins were shown to not only have inhibitory action against complement activation, but DAF-Ig also was shown to have a longer half-life than solution DAF. Molecules like this were made using several different complment regulatory proteins (CRPs) attached the Fc fragment. More study will be needed to determine which Fc donor isotype functions best. However, all of these CRP-Ig molecules have potential therapeutic uses for inflammatory disorders (Harris et al., 2002).
References
Ahmed SR, Lidington EA, Ohta R, Okada N, Robson MG, Davies KA, Leiges M, Harris CL, Haskard DO, Mason JC. (2003) Decay-accelerating factor induction by tumor necrosis factor-alpha, through a phosphatidylinosol-3 kinase and protein kinase C-dependent pathway, protects murine vascular endothelial cells against complement deposition. Immunology. 110:11153.
Fecke W, Long J, Richards A, Harrison R. (2002) Protection of hDaF-transgenic porcine endothelial cells against activation by human complement: role of the membrane attack complex. Xenotransplanation. 9:97.
Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. (2003) Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Molecular Immunology. 40:109-123.
Girardi G, Bulla R, Salmon JE, Tedesco F. (2006) The complement system in the pathophysiology of pregnancy. Molecular Immunology. 43:68-77.
Harris C, Abbott R, Smith R, Morgan B. (2005) Molecular dissection of interactions between components of the alternative pathway of complement and decay accelerating factor (CD55). Journal of Biological Chemistry. 280(4):2569-2578.
Harris CL, Williams AS, Linton SM, Morgan BP. (2002) Coupling complement regulators to immunoglobulin domains generates effective anti-complement reagents with extended half-life in vivo. Clinical Experimental Immunology. 129:198-207.
Hasan RJ, Pawelczyk E, Urvil PT, Venkatarajan MS, Goluszko P, Kur J, Selvarangan R, Nowicki S, Braun WA, Nolwicki BJ. (2002) Structure-function analysis of decay-accelerating factor: identification of residues important for binding of the Escherichia coli Dr adhesin and complement regulation. Infection and Immunity. 70(8):4485-4493.
Janeway, CA, Travers P, Walport M, Shlomchik MJ. (2005) Immunobiology: The immune system in health and disease, 6th edition. New York: Garland Publishing.
Kim DD and Song W. (2006) Membrane complement regulatory proteins. Clinical Immunology. 118:127-136.
Kuttner-Kondo LA, Mitchell L, Hourcade DE, Medof ME. (2001) Characterization of the active sites in decay-accelerating factor. Journal of Immunology. 167:2164-2171.
Lin F, Emancipator SN, Salant DJ, Medof ME. (2002) Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 82(5):563-569. (abstract)
Lin F, Kaminski H, Conti-Fine B, Wang W, Richmonds C, Medof ME. (2002) Markedly enhanced suscepitibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. Journal of Clinical Investigation. 110(9):1269-1274.
Lukacik P, Roversi P, White J, Esser D, Smith GP, Billington J, Williams PA, Rudd PM, Wormald MR, Harvey DJ, Crispin MDM, Radcliffe CM, Dwek RA, Evans DJ, Morgan BP, Smith RA. (2004) Complement regulation at the molecular level: The structure of decay-accelerating factor. PNAS. 101(5):1279-1284.
Mason JC, Ahmed Z, Mankoff R, Lidington EA, Ahmed S, Bhatia V, Kinderlerer A, Randi AM, Haskard DO. (2002) Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circulation Research. 91:696-703.
Mason JC, Steinberg R, Lidington EA, Kinderlerer AR, Ohba M, Haskard DO. (2004) Decay-accelerating factor induction on vascular endothelium by vascular endothelial growth factor (VEGF) is mediated via a VEGF receptor-2 (VEGF-R2)- and protein kinase C-α/ε-dependent cytoprotective signaling pathway and is inhibited by cyclosporin A. Journal of Biological Chemistry. 279(40):41611-41618.
Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T. (1994) Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal mocturnal hemoglobinuria. New England Journal of Medicine. 330:249.
Medof M, Walter E, Rutgers J, Knowles D, Nussenzweig V. (1987) Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165:848-864.
Morgan BP and Holmes CH. (2000) Immunology of reproduction: protecting the placenta. Current Biology. 10:R381-R383.
Parker C, Omine M, Richards S, Nishimura JI, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socie G. (2005) Diagnosis and mangament of of paroxysmal nocturnal hemoglobinuria. Blood. 3699-3709.
Saadi S and Platt JL. (1998) Immunology of xenotransplantation. Life Science. 62:763.
Stuart AD, Eustace HE, McKee TA, Brown TDK. (2002) A novel entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. Journal of Virology. 76(18):9307-9322.
Uhrinova S, Lin F, Ball G, Bromek K, Uhrin D, Medof E, Barlow P. (2003) Solution structure of a functionally active fragment of decay-accelerating factor. PNAS. 100(8):4718-4723.
Williams P, Chaudhry Y, Goodfellow I, Billington J, Powell R, Spiller O, Evans D, Lea S. (2003) Mapping CD55 function. Journal of Biological Chemistry. 278(12):10691-10696.