This web page was produced as an assignment for an undergraduate course at Davidson College.
CD154 (CD40L)
Structure
Figure 1: CD154 molecule. Notice the beta sheet structure. Image courtesy of Protein Data Bank.
CD154 is a member of the tumor necrosis factor family and is present as a costimulatory molecule on T cells. The gene encoding CD154 lies on the long arm of the X chromosome (Etzoni and Ochs, 2004). The CD154 molecule is composed almost entirely of beta sheets.

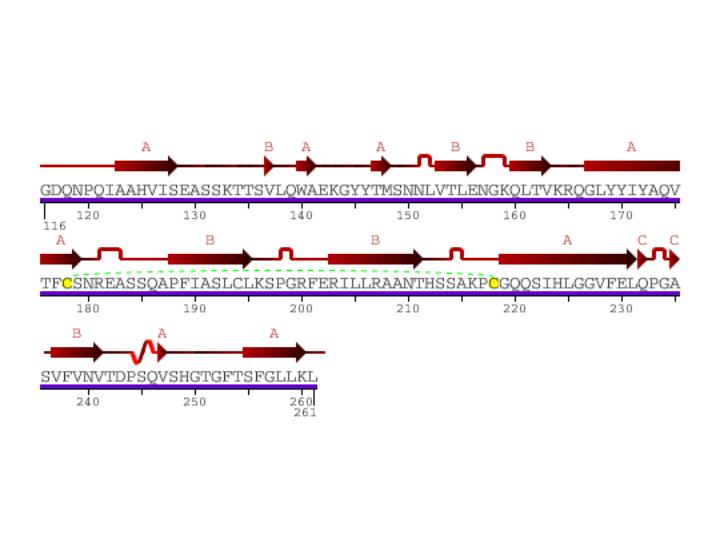
Figure 2: Secondary structure of CD154. Arrows represent beta sheets. Kinks show turns in secondary structure. Yellow letters linked by green dashed lines show sulfur bonds. S-shaped curve shows a 310 helix. Image is courtesy of Protein Data Bank.
Besides one small 310 helix, CD154 has three beta sheets, each composed of multiple beta strands. This protein has a transmembrane region with both an extra cellular region and a cytoplasmic tail. Such attributes facilitate the signaling function of CD154. In vivo CD154 forms a trimer as pictured below.
Figure 3: CD154 trimer. Image courtesyof Protein Data Bank
It is the trimerization of these three subunits, induced by binding to CD40, that causes both the T cell and the target cell to begin signal transduction that results in activation.
Function
CD154, along with its receptor, CD40, are involved in signaling between CD4-positive cells and B cells, macrophages, epithelial cells, and platelets. In B cells, the binding of CD154 to CD40 causes target B cells to undergo isotype switching and growth, thereby aiding CD4-positive cells in the activation of B cells (Janeway et. al, 2005). It has also been suggested that CD154 plays a role in positive and negative selection of the various VH genes in memory B cells, thereby determining which VH genes are expressed (Breinschek et al., 2000).
The CD154/CD40 interaction also participates in the activation of macrophages by TH1 cells. CD154 binding causes macrophages to be more sensitive to IFN-γ, a macrophage activating factor. In response to this interaction with the T cell, the macrophage also increases its production of B7 co-stimulatory molecules and MHC class II peptide presentation molecules (Janeway et. al, 2005). This pattern of upregulation enables more CD4-positive cells to be activated.
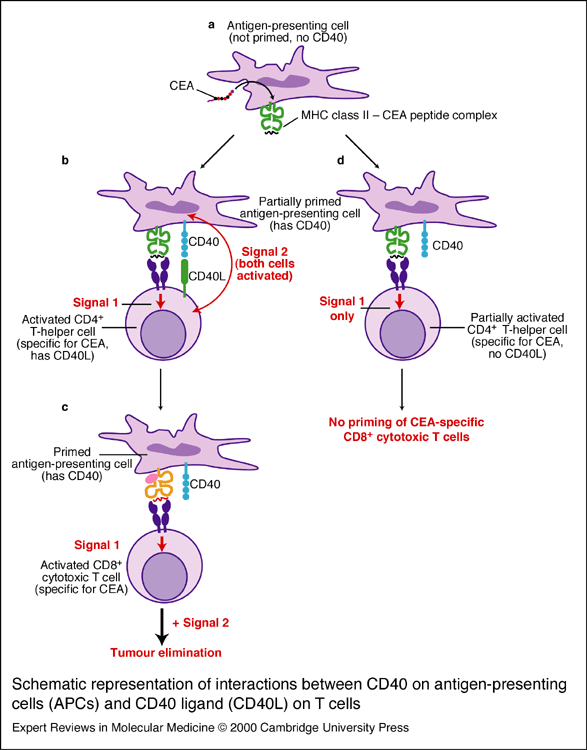
Figure 4: Role of CD40/CD154 interaction in T cell and macrophage activation. Antigen presenting cells (APC) require two stimuli to become activated (a). The first signal is transmitted upon the binding of a peptide presented in the context of a MHC class II molecule and the CD4 positive T cell receptor (b). If only one of these signals is received, the APC is not activated (c). In this case, the activated APC can continue on to prime cytotoxic T cells for the purpose of tumor elimination (d). Image taken from Horig et al., 2000 (permission pending).
Soluble CD154 (sCD154) has also been implicated in stabilizing interactions between adjacent platelets (OMIM). The mechanism for this is unknown; however, it has been shown that sCD154 acts as a ligand for some platelet agonist proteins (Andre et al., 2002). There is a concomitant release of sCD154 by CD4-positive T cells and stimulation of platelets following lymphocyte activation. Besides its role in the stabilization of platelets, sCD154 is thought to promote inflamation and coagulation (Andre et al., 2002).
What happens without CD154?
Mutations in CD154 have been implicated in hyper IgM syndrome type 1. This version of hyper IgM (HIGM) is transmitted as an X-linked genetic disease. In the absence of CD154, B cells are unable to undergo isotype switching, causing immunodeficiencies in the patients with HIGM1 (OMIM). Thus, one result of HIGM1 is the increased amount of circulating IgM antibodies, while levels of IgG, IgA, and IgE are decreased. Patients with HIGM1 often develop respiratory and digestive problems as a result of opportunistic pathogens (Etzoni and Ochs, 2004).
An animal model of HIGM has been created using gene targeting in mice (Xu et al., 1994). Mice defficient in CD154 experience a decreased thymus-dependent response to antigens, while thymus-independent reactions are unchanged. Also, lymphoid organs show now germinal centers, which suggests that B cells are unable to make memory cells in response to antigen.
An absence of CD154 could also be associated with a weakened thrombus. Moreover, a build up of sCD154 may increase a patient's risk of thrombosis of arteries in areas subject to high stress (Andre et al., 2002).
How can CD154 be used in treatments?
Because the absence of CD154 can cause instability in the thrombus, one possible application of CD154 in medicine is blocking this molecule when stable accumulations of thrombi is destructive. One such case is following a cardiovascular intervention (Andre et al., 2002). Therefore, inhibition of CD154 could render any aggregations of thrombi instable, decreasing the risk of reoccurence of thrombosis following heart surgery.
Blocking CD154 has also shown to be successful in curbing the immune response to allografts in mice (Xu et al., 2006). In this case, the blockage of CD154 could act clinically as an immunosupressant in individuals undergoing transplants.
Works Cited
Andre, P., Srinivasa Prasad, K.S., Denis, C.V., He, M., Papalia, J.M., Hynes, R.O., Phillips, D.R., Wagner, D.D. 2002. CD40L stabilizes arterial thrombi by a β3 integrin-dependent mechanism. Nature Medicine 8(3):247-252.
Brezinschek, H., Dorner, T., Monson, N.L., Bresinschek, R.L., Lipsky, P.E. 2000. The influence of CD40-CD154 interactions on the expressed human VH repertoire: analysis of VH genes expressed by individual B cells of a patient with X-linked hyper-IgM syndrome. International Immunology 12(6):767-775.
Etzioni, A., and Hans D. Ochs. 2004. The hyper IgM syndrome--an evolving story. Pediatric Research 56(4):519-525.
Hörig, H., Medina, F.A., Conkright, W.A., and Kaufman, H.L. 2000. Strategies for cancer therapy using carcinoembryonic antigen vaccines. Exp. Rev. Mol. Med. http://www.expertreviews.org/0000168Xh.htm
Janeway, C.A., Travers, P., Walport, M., Shlomchik, M.J. 2005. Immunobiology. New York. Garland Science.
Online Mendelian Inheritance in Man, OMIM (TM). Johns Hopkins University, Baltimore, MD. MIM Number: 308230: 2005: http://www.ncbi.nlm.nih.gov/omim. Accessed February 2006.
PDB ID: 1ALY. Karpusas, M., Hsu, Y.M., Wang, J.H., Thompson, J., Lederman, S., Chess, L., Thomas, D. 1995. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure 3:1031-1039.
Xu, H., Zhang, X., Mannon, R.B., and Kirk, A.D. 2006. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. Journal of Clinical Investigations 116:769-774.
Xu, J., Foy, T.M., Laman, J.D., Elliott, E.A., Dunn, J.J., Waldschmidt, T.J., Elsemore, J., Noelle, R.J., Flavell, R.A. 1994. Mice deficient for the CD40 ligand. Immunity 1(5):423-431.
If you have any questions or concerns about this web page please contact Sarah.
Return to Davidson College Biology Home Page.
Return to Immunology Home Page.
Return to Sarah's Immunology Home Page.