Introduction: Terminal deoxynucleotidyl transferase (TdT) is a nuclear enzyme responsible for the template-independent addition of N-nucleotides at gene segment junctions of developing lymphocytes (Komori et al., 1993). Lymphocytes differentiate in the bone marrow and the thymus, for B cells and T cells respectively, and a key component of their development involves somatic recombination of variable gene segments. Regulated rearrangement of the antigen-specific lymphocyte receptor gene segments through genetic recombination augments the combinatorial diversity of the antigen-specific receptor repertoire. V(D)J recombinase, an enzyme complex that includes the protein products of the RAG-1 and RAG-2 genes, regulates somatic recombination by selectively binding recombination signal sequences (RSS) in a specific 12/23 linkage rule based on spacer length. The 12/23 rule ensures that the gene segments are ligated in the correct arrangement, specifically V-D-J for T-cell receptor (TCR) β chains and B-cell receptor (BCR) heavy chains and V-J for TCR α chains and BCR light chains. After recognizing complementary RSS's and uniting the two segments to be joined, the RAG protein complex mediates random double-stranded breaks, which forms hairpin structures at the RSS coding sequence. At this junction, TdT can add N-nucleotides to available 3' hydroxy ends of the hairpin, thus, generating highly variable coding joints (Janeway et al, 2005). TdT adds from 2-5 base pairs per coding joint, with a bias towards G-C pairing (Mickelsen et al., 1999). In TdT-knockout mice, the occurance of homology-mediated joins based on specific germline encoded nucleotide pairing is enhanced (Komori et al., 1993). In contrast, the addition of N-nucleotides by TdT leads to junctional diversity at the coding joints between gene segments. Studies on TdT-deficient lymphocytes demonstrate no N-nucleotide addition in the variable region of the lymphpocyte antigen-specific receptors. TdT-knockout mice had a ten-fold reduction of TCR variation compared to WT mice, showing that the addition of N-nucleotides contributes to almost 90% of TCR diversity. In WT lymphocytes, the addition of N-nucleotides to the coding joints that encode the CDR3 region of the receptor are specifically selected for during development (Cabaniols et al., 2001). By increasing the diversity of the antigen-binding region of the lymphocyte receptor, N-nucleotide addition allows lymphocytes to recognize a diverse array of pathogens. Therefore, while TdT is not essential for somatic recombination, as shown in TdT-knockout mice that are still immuno-competent, TdT is of critical value for antigen-specific receptor diversity (Cabaniols et al., 2001).
Structure and Function: TdT has been extensively characterized and defined as a 58 kMW tissue-specific enzyme expressed during BCR and TCR rearrangements. TdT adds non-germline N-nucleotides to double-stranded receptor gene segment DNA, which increases the diversity of the receptor binding specificities (Peralta-Zaragoza et al., 2004). Specifically, TdT belongs to the pol X family of polymerase molecules. This subgroup of the nucleotidyltransferase family does not require a template for elongation and shares common structural homology of the catalytic center, C-terminal (BRCT) domains, and helix-hairpin-helix domains (HhH). Crystallography methods have determined that TdT has structural similarity to polymerase B including conserved amino acids in the catalytic active site, but the function of TdT is unique (Repasky et al., 2004).
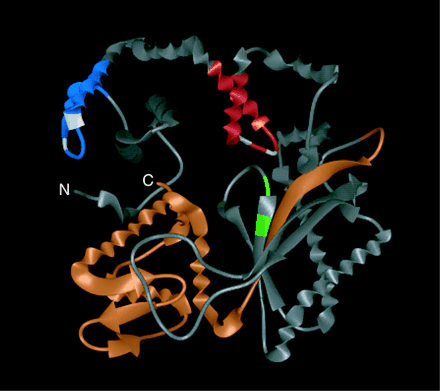
The C-terminus and HhH2 sequences are most important to TdT funtion. In mouse mutants that have deletions in these structural regions, a loss of TdT function is observed and no N-nucleotides are added. This loss of function can be due to misfolding of the protein, reduced expression, or because of a direct effect on the catalytic activity of the molecule. The C-terminus, also referred to as BRCT, seems to play a role in helping TdT home to the coding joint during somatic recombination and remain there. Also, the active form of TdT requires it to attain a closed structural conformation, which occurs due to specific interactions between the C-terminus and N-terminus. The HhH2 is responsible for the alignment of the coding joint DNA near the active site of the TdT protein and, therefore, is functionally important (Repasky et al., 2004).
Alternative splicing of the TdT gene produces two structural isotypes that vary in the length of the protein. The long isotype, named TdTL, has a 60 bp insertion that is not present in the short, TdTS variant. The functional differences between the two isotypes is still trying to be elucidated, but some studies have already revealed interesting data. One study found a 34% reduction in N-nucleotide addition when a mixture of TdTL and TdTS was expressed in mice lymphocytes (Benedict et al., 2000).This data shows that TdTL may down regulate N-nucleotide addition catalyzed by TdTS. Another study found that a mixture of TdTL and TdTS reduced the average length of N-nucleotide addition. Because of this, TdTL is hypothesized to have 3' to 5' exonuclease activity, which biochemical studies have also verified (Repasky et al., 2004).
Expression and Regulation: Immunofluorescence analysis has shown that TdT is present in the nucleus of immature lymphocytes and leukemic cells, but that it is not found in non-lymphoid cells (Komori et al., 1993). TdT is expressed throughout the rearrangement of TCR α and β polypeptide chains and is also expressed during the heavy chain rearrangement of BCR's. Because of this, N-nucleotides are preferentially found at the coding joints between these gene segments. This influences gene segment choice and alters the DNA reading frame, thus, increasing the diversity of the antigen receptors of these B cells and T cells. TdT concentrations in adult lymphocytes far exceed TdT concentrations in fetal lymphocytes, showing that transcriptional expression is tightly regulated. TdT is first detected in the thymus four days after birth (Cabanioles et al., 2001). During fetal development before TdT is expressed, the joining of gene segments is mediated by homology-pairing of specific nucleotide sequences. This germline-mediated binding provides a window in development where lymphocytes with clonally-specific receptors develop and provide the protective immunity needed in that life stage (Benedict et al., 2000). About a week after birth, increased levels of cAMP and cGMP up-regulate TdT, which causes the diversity of the TCR and BCR repertoire to increase as well. The expression of TdT in these lymphocytes, however, is still under the influence of various transcription factors. For instance, the activation of PKC, which induces the synthesis of the Fos and Jun subunits of the AP-1 transcription factor, down-regulates TdT expression. AP-1 regulates TdT transcription by binding to TdT promoter regions of DNA and, thus, excluding TdT from binding. This mechanism helps explain why non-lymphoid cells do not produce TdT (Peralta-Zaragoza et al., 2004). Another mechanism to explain this observation correlates the amount of TdT expression to the amount of methylation of the TdT gene. The methylation of unused genes in certain tissues is a common mechanism that our cells use to regulate the proteins that are made. Studies of the thymus have shown that high levels of TdT expression are associated with complete demethylation of the HhH1 promoter regions (Nourrit et al., 1999). This study used Southern Blot analysis to characterize the -111 to +58 nucleotide promoter region of TdT and concluded that promoter demethylation helps control TdT transcription. This study determined that lymphocytes expressing TdT have either no methylation or partial methylation of the TdT promoter, while in non-lymphoid cells the promoter region has complete methylation (Nourrit et al., 1999). Differences in methylation might help explain why there is a paucity of N-nucleotides found in the coding joints of BCR light chains. Also, DNA-dependent protein kinase (DNA-PK) has been shown to co-localize with TdT on the coding joints of DNA. Researchers studying this direct interaction between DNA-PK and TdT believe that the TdT can form a stable complex with the DNA-PK , which then can regulate TdT activity by controlling the length and composition of N-nucleotide additions (Mickelsen et al., 1999). It is known that Ku heterodimers regulate the activation of DNA-PK, so future research will focus on the complex relationship between DNA-PK, Ku, and TdT.
Clinical Applications and Future Research: If T cell development occurs in the thymus without TdT expression, many TCR's expressed on the cell surface will contain the same germline-encoded gene segments. These clonal receptors can feasibly become cross-reactive against self-molecules and, thus, increase autoimmunity (Cabaniols et al., 194). Substantitive research, however, is lacking in this area. Other researchers, in contrast, have found that TdT-knockout mice are usually healthy. In these mice, they observed that the TCR's were more "promiscuous" with regard to peptide recognition, that TCR positive selection occurred, and that the BCR was less polyreactive (Feeney et al., 2001). These researchers then compared the acuteness of the autoimmune disease lupus erythematosus to N-nucleotide addition in T cells and B cells. They looked at both TdT and Fas deficient lupus murine models and concluded that gene knockouts had longer life spans, decreased skin lesions, and lower levels of autoimmune antibodies, like anti-dsDNA. They believe that the receptor repertoire determines autoimmunity effects of lupus. They believe that the expression of TdT increases the chance of accumulating nucleotide additions that enhance autoimmunity by changing the amino acid composition and the reading frame (Feeney et al., 2001). Therefore, there is a definite trade-off between increasing the receptor repertoire to recognize many pathogens, while at the same time increasing the chances of recognizing a self-antigen. While this study specifically showed that the acute form of lupus requires the addition of N-nucleotides to T cell and B cell repertoires, this data may be relevant to the study of other autoimmune diseases. Additional research in this area is necessary and should be encouraged. Other questions that remain unanswered deal with the enzymatic role of TdTL, its function in relation to TdT, and a comprehensive understanding of the effect of TdT on T cell and B cell repertoires.
Benedict, Cindy L., et al. "Terminal Deoxynucleotidyl Transferase and Repertoire Development." Immunological Reviews 175 (2000): 150-157.
Bentolila, Laurent A., et al. "Extensive Junctional Diversity in Ig Light Chain Genes from Early B Cell Progenitors of microMT Mice." The Journal of Immunology 162 (1999): 2123-2128.
Cabaniols, Jean-Pierre, et al. "Most α β T Cell Receptor Diversity Is Due to Terminal Deoxynucleotidyl Transferase." Journal of Experimental Medicine 194.9 (2001): 1385-1390.
Feeney, Ann J., et al. "Terminal Deoxynucleotidyl Transferase Deficiency Decreases Autoimmune Disease in MRL-Fas Mice." The Journal of Immunology 167 (2001): 3486-3493.
Janeway, C.A., Travers, P., Walport, M., Schlomchik, M. Immunobiology 6th Ed: The Immune System in Health and Disease. New York: Garland Publishing, 2005.
Klonowski, Kimberly D. and Marc Monestier. "Heavy Chain Revision in MRL Mice: A Potential Mechanism for the Development of Autoreactive B Cell Precursors." The Journal of Immunology 165 (2000): 4487-4493.
Komori, Toshihisa, et al. "Lack of N Regions in Antigen Receptor Variable Region Genes of TdT-Deficient Lymphocytes." Science 261.5125 (1993): 1171-1175.
Marshall, Aaron J., et al. "Terminal Deoxynucleotidyl Transferase Expression During Neonatal Life Alters Dh Reading Frame Usage and Ig-Receptor-Dependent Selection of V Regions." The Journal of Immunology 161 (1998): 6657-6663.
Mickelsen, Scott, et al. "Modulation of Terminal Deoxynucleotidyltransferase Activity by the DNA-Dependent Protein Kinase." The Journal of Immunology 163 (1999): 834-843.
Nouritt, Francoise, et al. "Mthylation of the Promoter Region May be Involved in Tissue-Specific Expression of the Mouse Terminal Deoxynucleotidyl Transferase Gene." JMB 222 (1999): 217-227.
Peralta-Zaragoza, Oscar, et al. "Terminal Deoxynucleotidyl Transferase is Down-Regulated by AP-1-like Regulatory Elements in Human Lymphoid Cells." Immunology 111 (2004): 195-203.
Repasky, Jamie A.E., et al. "Mutational Analysis of Terminal Deoxynucleotidyltransferase-Mediated N-Nucleotide Addition in V(D)J Recombination." The Journal of Immunology 172 (2004): 5478-5488.
Wei, Chiju, et al. "Murine Pro-B Cells Require IL-7 and Its Receptor Complex to Up-Regulate IL-7R, Terminal Deoxynucleotidyltransferase, an cμ Expression." The Journal of Immunology 164 (2000): 1961-1970.