Back to Dan Heeren's Immunology Homepage
Elephantiasis is technically the hardening and thickening of the skin following swelling of the arms, breasts, legs, or for men the genitals known as lymphedema (CDC, 2004). Parasitic nematodes that cause this condition can be Wuchereria bancrofti, Brugia malayi, or Brugia timori, but W. bancrofti is by far the most common (Wikipedia, 2006). These worms are carried my various nematode-specific species of mosquitoes including Culex, Aedes, and Anopheles for W. bancrofti. The mosquitoes take a blood meal from a human and at the same time introduce the larval nematode, which moves into the lymphatic system and there grows to adulthood, reaching a length of one to four inches and living for an average of seven years, but in one case living forty years. A male and a female mate and produce millions of microscopic larvae which move to the blood stream. Another mosquito takes a blood meal and transports the larval worm to another person (Placek, 2002). It is still unclear if the disease is caused by the blockage of the lymphatics by the nematode worm or by the immune response to the worm and its symbiotic bacteria Wolbachia (Wikipedia, 2006).
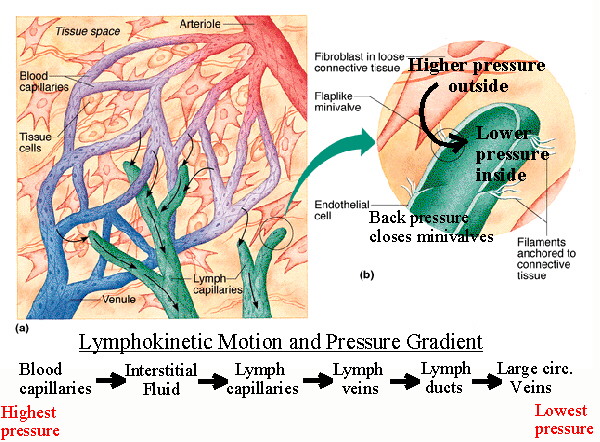
Figure 1: The lymph system at the capillary bed. When fluids “leak” out of the capillary beds, the lymph system takes it up and returns it to circulation. If a nematode plugs up the lymphatic, the fluid from the tissue cannot be removed, leading to extreme swelling (edema). Image courtesy of Jim Swan. Original image copyright Benjamin Cummings Publishing Company.
The infection of the lymph vessels by a nematode is known as lymphatic filariasis, but it has also been known by names such as Barbados leg, elephant leg, morbus herculeus, mal de Cayenne, and myelolypmhangioma (Placek, 2002). This should not be confused with Proteus syndrome, the disorder partly responsible for the deformity of the “Elephant Man” Joseph Merrick (Wikipedia, 2006). Lymphatic filariasis affects over 120 million people in the tropics and sub-tropics of Asia, Africa, the Western Pacific, and parts of Central and South America. This disease has existed in humans for centuries, as there are references in ancient Persian and Indian texts to elephant-like swellings of the extremities and genitals (Placek, 2002). Usually, a person needs to have multiple exposures to larval nematodes to develop the condition (CDC, 2004).
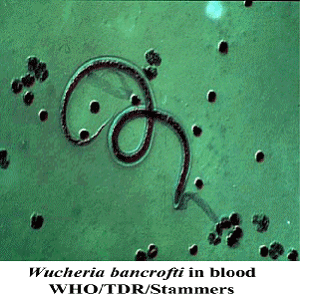
Figure 2: Wucheria bancrofti. This parasitic nematode is the main worm that infects the lymphatics and causes lymphatic filariasis. Permission pending from filariasiscenter.org.
Interestingly, there are also rare cases of elephantiasis in people that are not infected by a nematode. Nonfilarial elephantiasis, or podoconiosis, generally occurs in the mountains of central Africa, and is caused by the volcanic ash that works its way through people’s bare feet and into their lymphatics. They then either block them or initiate an immune reaction, leading to elephantiasis (Wikipedia, 2006).
Gross impact on the immune system
The lymph system drains tissues of the extracellular fluid that “leaks” out of capillaries and eventually returns it to circulation. First, however, the lymphatics take the lymph to lymph nodes where it can be screened for pathogens.
When the worms block the lymphatics, the body initiates an allergic reaction, with symptoms including fever, chills, shaking, sweating, headaches, vomiting, and localized pain. Additionally, there may be red streaking, joint pain, enlargement of the lymph nodes, and skin ulcers. At first, the affected area may feel soft, but the skin eventually hardens. It may become warty and cracked, allowing bacteria to infect the open wounds, complicating the disease (Placek, 2002). It is thought that the lymphatic damage leads to impaired lymph flow, allowing favorable conditions for secondary bacterial infections. These cause even more vessel damage, worsening the lymphedema (Baird et al., 2002). There is evidence that a protein secreted by the nematode can actually stimulate the B cells nearby. This signal is not enough to activate them, but instead desensitizes them to any further activation signals (Deehan et al., 1998). Few immune system cells can reach the infected lesions, and the ones that do simply cause more inflammation or are rendered ineffective (Nookala et al., 2004). Ultimately, because the lymphatics are blocked, either by the worm or by localized inflammation, fluid is unable to drain out of the tissue. Back pressure causes dilation of superficial vessels, resulting in extreme swelling. Obstructed blood supply leads to gangrenous tissue because tissue can’t receive oxygen or lose carbon dioxide (Kinney, 2004).
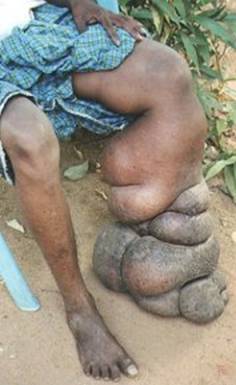
Figure 3: A severe case of Elephantiasis in the left leg. Note the extreme swelling and the warty, hard skin. This condition is extremely painful and may eventually have to be amputated. Photo courtesy of lepra.org/uk.
Take a closer look
The normal immune response includes a T helper 2 (Th2)-mediated activation of B cells. The activated T cells release the cytokine interleukin-4 (IL-4), which skews the antigen-specific antibody response to IgE (Yazdanbakhsh et al., 2005). As IgE returns to the site of infection, it binds the antigen, and then binds the high-affinity Fc receptors on mast cells, causing them to degranulate. Mast cells release histamines and other inflammatory mediators which attract other inflammatory cells such as macrophages, eosinophils, and dendritic cells (Janeway et al., 2005). It is thought that the IgE is produced by the live adult nematode, but when it breaks down, internal proteins cause the generation of IgG3 antibodies. In a subset of the population that is infected with the nematode but shows no symptoms of elephantiasis, there is little IgE or IgG3, causing scientists to believe that it may very well be the immune response to the infection that causes the most damage. In fact, some autoimmune diseases such as systemic lupus erythematosus have been linked to high levels of IgG3 (Yazdanbakhsh et al., 2005).
We have seen that there is a potentially dangerous antibody response to an antigen associated with the infection by the nematode, but what exactly is that antigen? There is debate as to whether it is an external part of the worm, some internal protein of the worm, or part of the Wolbachia bacteria. There are dramatically higher levels of IgG specific for a surface protein on Wolbachia in those individuals showing lymphedema. This strongly suggests that the dramatic immune response is to the Wolbachia that are exposed upon the death of the adult worm. Antibiotics specific for Wolbachia have decreased both larval nematode load and the development of the worms. It also renders adult female worms infertile. It has also been shown that drugs that kill adult nematodes result in an increased disease state, possible because of the release of the Wolbachia bacteria (Punkosdy et al., 2003; Gopinath et al., 2000). It has been theorized that the degradation of the adult worm exposes the Wolbachia, which switches the response to a Th1-type response, the primary response (Punkosdy et al., 2003).
In reactions that showed lymphedema, there were activated T cells and Natural Killer (NK) cells that secreted interferon-gamma (IFN-γ) under the influence of IL-12 from macrophages (King et al., 1993). This causes inflammation at the site of infection, leading to further recruitment of inflammatory mediators and localized clotting. Inflammation throughout the limb leads to the swelling. This swelling causes the lack of blood flow which allows for opportunistic infection and gangrene. The infected individuals who don’t show symptoms generally have highly increased levels of IL-10 released from T cells and macrophages that cause the suppression of macrophages (Mahanty et al., 1997). It appears that the IL-10 decreased MHC class II expression on antigen presenting cells (King et al., 1993).
Factors affecting pathology
Poor sanitation and rapid, dense growth has led to areas that are prime breeding and feeding grounds for mosquitoes in poorer nations (CDC, 2004). Areas that are less sanitary will contribute to secondary infections of the lesions and increase the amount of lymphedema. Interestingly, there is evidence that if a pregnant woman is infected with W. bancrofti, her child is three to four times as likely to be infected as well. There is no correlation between father and child, showing that indeed there is a maternal impact. W. bancrofti antigen likely passes through the fetus’ circulation while in utero, and the child develops a tolerance to it. Later in life, the nematode can more easily infect the person because there are fewer nematode-specific lymphocytes and there will be less cytokine production (Malhotra et al., 2003).
Treatments and prevention
First and foremost, prevention is the key to good health. Always sleep under a mosquito net. The mosquitoes feed at night, and perhaps not coincidentally, that is when larval nematodes move out to the peripheral circulation. It is for this reason that blood tests are either done at night or after giving a “provocative” dose of medication to encourage the larvae to move out into the bloodstream. Also, the reduction of the local mosquito population can reduce infection rates.
Once a person is infected, the drug of choice is diethylcarbamazine (DEC). DEC kills the larvae quickly, but barely kills any of the adults. Therefore, multiple courses are required to kill off larvae while the adults die on their own (Placek, 2002). In addition, simple doxycycline may be an effective aide as it kills the Wolbachia (Wikipedia, 2006). Without medication, one can only wash the swollen area with soap and water to control the opportunistic infections and elevate and exercise the limb to keep the fluid and lymph moving (CDC, 2004).
References
Baird J, Charles J, Streit T, Roberts J, Addiss D, Lammie P. 2002. Reactivity to Bacterial, Fungal, and Parasite Antigens in Patients with Lymphedema and Elephantiasis. American Journal of Tropical Medicine and Hygiene 66: 163-169.
Centers for Disease Control and Prevention. 2004 September 27. Lymphatic Filariasis Fact Sheet. <http://www.cdc.gov/ncidod/dpd/parasites/lymphaticfilariasis/2004_PDF_LymphaticFilariasis.pdf> Accessed 2006 March 22.
Deehan M, Frame M, Parkhouse R, Seatter S, Reid S, Harnett M, Harnett W. 1998. A Phosphorylchlorine-Containing Filarial Nematode-Secreted Product Disrupts B Lymphocyte Activation by Targeting Key Proliferative Signaling Pathways. The Journal of Immunology 160: 2692-2699.
Gopinath R, Hanna L, Kumaraswami V, Perumal V, Kavitha V, Vijayasekaran V, Nutman T. 2000. Perturbations in Eosinophil Homeostasis following Treatment of Lymphatic Filariasis. Infection and Immunity 68: 93-99.
Janeway C, Travers P, Walport M, Shlomchik M. 2005. Immunobiology: the immune system in health and disease, 6th edition. Garland Science Publishing: New York.
King C, Mahanty S, Kumaraswami V, Abrams J, Regunathan J, Jayaraman K, Otteses E, Nutman T. 1993. Cytokine Control of Parasite-specific Anergy in Human Lymphatic Filariasis. The Journal of Clinical Investigation 92: 1667-1673.
Kinney J. 2004 May 7. Elephantiasis. <http://elephantiasis.freeyellow.com/print.html> Accessed 2006 March 22.
Mahanty S, Ravichandran M, Raman U, Jayaraman K, Kumaraswami V, Nutman T. 1997. Regulation of Parasite Antigen-Driven Immune Responses by Interleukin-10 (IL-10) and IL-12 in Lymphatic Filariasis. Infection and Immunity 65: 1742-1747.
Malhotra I, Ouma J, Wamachi A, Kioko J, Mungai P, Njzovu M, Kazura J, King C. 2003. Influence of Maternal Filariasis on Childhood Infection and Immunity to Wuchereria bancrofti in Kenya. Infection and Immunity 71: 231-5237.
Nookala S, Srinivasan S, Kaliraj P, Narayanan R, Nutman T. 2004. Impairment of Tetanus-Specific Cellular and Humoral Responses following Tetanus Vaccination in Human Lymphatic Filariasis. Infection and Immunity 72: 2598-2604.
Placek C. 2002 December. Elephantiasis. <http://www.healthatoz.com/healthatoz/Atoz/ency/elephantiasis_pr.jsp> Accessed 2006 April 23.
Punkosdy G, Addiss D, Lammie P. 2003. Characterization of Antibody Response to Wolbachia Surface Protein in Humans with Lymphatic Filariasis. Infection and Immunity 71: 5104-5114.
Wikipedia. 2006 April 24. Elephantiasis. <http://en.wikipedia.org/wiki/Elephantiasis> Accessed 2006 March 22.
Yazdanbakhsh M, Paxton W, Brandenburg A, van Ree R, Lens M, Partono F, Maizels R, Selkirk M. 1995. Differential Antibody Isotype Reactivity to Specific Antigens in Human Lymphatic Filariasis: gp15/400 Preferentially Induces Immunoglobulin E (IgE), IgG4, and IgG2. Infection and Immunity 63: 3772-3779.
© Copyright 2006 Department of Biology, Davidson College, Davidson, NC 28035
Send comments, questions, and suggestions to: daheeren@davidson.edu