- This web page was produced as an assignment for an undergraduate course at Davidson College -
CD45
also known as Leukocyte Common Antigen
CD45 (leukocyte-common antigen, L-CA) is a transmembrane glycoprotein of the leukocyte-specific receptor-like protein tyrosine phosphatase (RPTP) family (Dios et al. 2005). It is expressed on all nucleated haematopoietic cells and is one of the most abundant cell surface glycoproteins, covering up to 10% of cell surface area (Hermiston et al. 2003). CD45 plays an important role in the positive regulation of antigen-receptor signaling in lymphocytes via the dephosphorylation of Src kinases, making CD45 essential for normal B and T lymphocyte development. CD45 can negatively control cytokine-receptor signaling by functioning as a Janus kinase (JAK) PTPase (Dios et al. 2005). Several isoforms of CD45 can be created by alternative splicing of exons 4, 5 and 6 of the extracellular domain. Isoform expression is cell-type specific and can influence biological function and alter biochemical-signaling events (Tchilian and Beverley 2006).
Like most members of the RPTP family, CD45 is comprised of a single transmembrane domain and a large cytoplasmic tail with two tandemly duplicated PTPase homology domains, D1 and D2. Only D1 has phosphatase activity and is required for the rescue TCR signaling in a CD45-deficient cell line (Hermiston et al. 2003). Studies have shown that the interaction between the membrane-distal domain and the spacer region separating the two tandem PTP domains is mediated by a stretch of amino acid residues in the carboxyl-terminal half of the spacer region. The membrane proximal region appears to function in stabilizing the interaction between the spacer region and the D2 domain without directly interacting with either D1 or D2 (Hayami-Noumi et al. 2000).
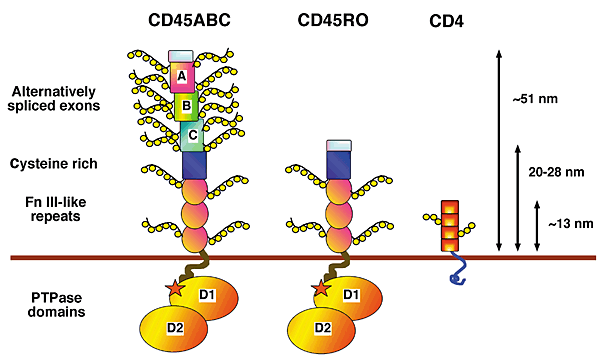
Alternative splicing of exons 4, 5, and 6 (designated A, B and C in figure 1) of the extracellular domain can generate up to eight CD45 isoforms, five of which are expressed at significant levels in T cells. CD45 isoform expression is cell type-specific and depends upon both the stage of differentiation and the state of activation of the cell (Tchilian and Beverley 2006). The three alternatively spliced exons encode multiple sites of O-linked glycosylation. As shown in Figure 1, the extracellular domain of CD45 isoforms differ substantially in size, shape and negative charge. Naïve T cells in humans express high-molecular-weight (240 kDa) CD45 isoforms that contain the A exon. Activation of these 'CD45RA' T cells causes a change in expression to low-molecular-weight (approx. 180 kDa) CD45RO isoforms associated with T cell memory and differentiated T cell function (Stanton et al. 2000; Tchilian and Beverley 2006). The remaining extracellular domain is heavily N-glycosylated and contains a cystein-rich region followed by three fibronectin type III repeats, as indicated by figure 1 (Penninger et al. 2001).

Figure 1. Alternative splicing of exons 4, 5 and 6 (designated A, B and C in figure) in the extracellular domain of CD45 creates various cell-type specific isoforms that differ in size, shape and negative charge (Penninger et al. 2001).
A primary function of CD45 is regulating the activation of Src family protein tyrosine kinase (Src-PTK) in lymphocytes. The regulation of antigen receptor signaling thresholds by CD45 is critical for the development of both B cells and T cells (Dios et al. 2005). In T cells, CD45 dephosphorylates the inhibitory C-terminal tyrosine residues of both p56lck and p59fyn tyrosine kinases. p56lck activation is an early and vital step in the signaling cascade that is initiated by TCR ligation. As illustrated in the upper panel of Figure 2, dephosphorylation of the negative regulatory tyrosine on Lck by CD45 produces a signal-competent pool of primed, active p56lck that can phosphorylate the TCR ITAMs if specific antigen is encountered. (Johnson et al. 2000). Csk (C-terminal Src kinase) antagonizes the action of CD45 by phosphorylating the inhibitory tyrosine at the negative regulatory site of Src kinases, as shown in figure 3. The reciprocal activity of CD45 and CSK maintains p56lck in a dynamic equilibrium between its inactive and active conformations (Hermiston et al. 2003). In B cells, CD45 functions similarly by modulating signaling thresholds and signal transduction through the BCR. CD45 differentially regulates BCR-induced activation of MAP kinases and can exert opposing effects on JNK and p38 at different stages of B cell maturation, controlling the B cell fate (Ogimoto et al. 2000).
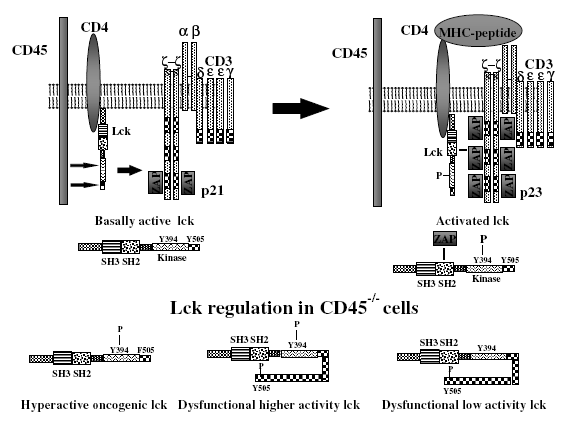
Figure 2. CD45 regulates T cell antigen signal transduction by dephosphorylating the tyrosine residue of p56lck. The upper panel depicts the molecular changes that occur upon ligation of the T cell receptor complex by the appropriate MHC:peptide complex. The lower panel shows the different phosphorylated species of p56lck that are often found in CD45-deficient cells (Alexander 2000).
Figure 3. Dynamic equilibrium between its active and inactive
conformation is maintained in Lck by the reciprocal activity of CD45 and Csk
(Hermiston et al. 2003). Through a variety of receptors, CD45 has been demonstrated to 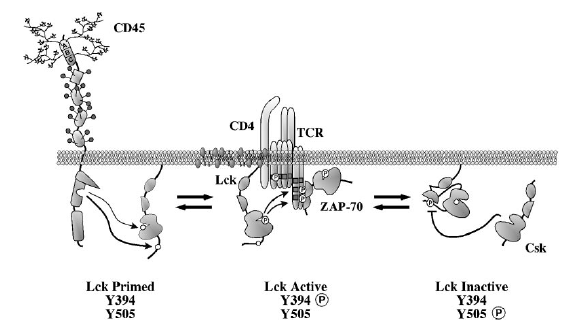
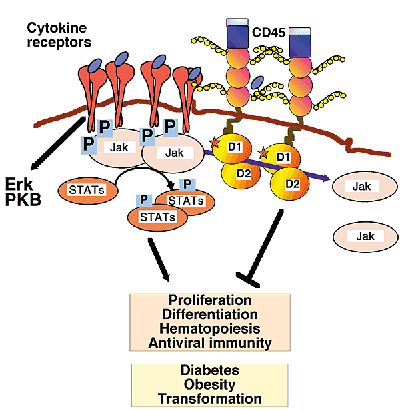
Figure 4. Upon stimulation, JAK cytokine receptors are phosphorylated on tyrosine residues and activated. Activated JAKs phosphorylate STATs, which function as transcriptional activators of the genes involved in cytokine expression. CD45 negatively regulates cytokine receptor-mediated signaling by acting as a Janus kinase (JAK) phosphatase. Loss of CD45 affects hematopoiesis, proliferation, differentiation and antiviral responses (Penninger et al. 2001).
It has been recently shown that CD45 may further modulate immune responses by regulating survival and apoptosis in immune cells. CD45-/- mice exhibit an increase in apoptosis, often as a result of ligation of CD45 with antibodies or galectin. Not all CD45 antibodies induce apoptosis, and the mechanism by which CD45 ligation triggers apoptosis is still unclear (Tchilian and Beverley 2006). Interestingly, several studies have shown that activated T cells upregulate B220 prior to apoptosis, although B220 is an isoform of CD45 normally found in B cells and a subset of NK cells (Hermiston et al. 2003).
The main role described for CD45 in macrophages is in the regulation of integrin-mediated adhesion. CD45 co-localizes with phosphotyrosine-containing proteins at focal adhesion sites, which it regulates by reducing the activity of the Src kinases p56/59lck and p53/56lyn (Roach et al. 1996). This down-regulates the signaling functions of at least one integrin, resulting in a reduction in macrophage adherence to the extracellular matrix and to other cells. Regulation of integrin-mediated adhesion by CD45 of is required for macrophage maturation and normal responses to environmental stimuli (Alexander 2000).
A model has been proposed for the negative regulation of CD45 function in which dimerization inhibits phosphatase activity through symmetrical interactions between the catalytic site and an inhibitory wedge containing the acidic residues. The total phoshpatase activity in a cell is determined by an isoform-determined equilibirum between CD45 monomers and dimers on the cell surface (Hermiston et al. 2000). This model was first developed when inhibition through mutual active-site occlusion was observed in the crystal structure of PTPase-alpha (Alexander 2000). Spontaneous and isoform-differential homodimerization (figure 5) is another mechanism that might regulate CD45 phosphatase activity. According to this model, CD45RO isoforms homodimerize more easily, leading to inhibition of phosphatase activity (Majetic et al. 2000). A third model proposes that the location of CD45 at the plasma membrane relative to other molecules may contribute to the regulation of CD45 (Alexander 2000).
The importance of regulating CD45 can be demonstrated by mice with a single point mutation that inactivates the inhibitory wedge of CD45. The mutation results in polyclonal lymphocyte activation, which causes lymphoproliferation, severe autoimmune nephritis with autoantibody production and death (Majeti et al. 2000).
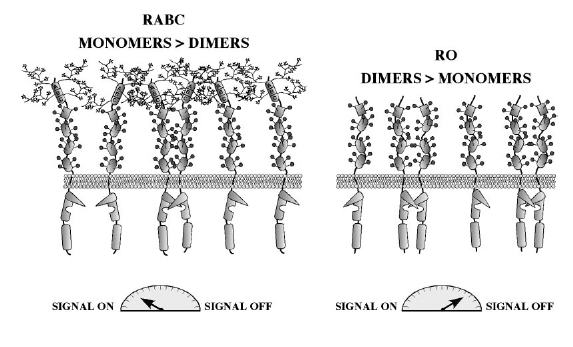
Figure 5. Isoform-differential dimerization may negatively regulate CD45. Large RA+ isoforms (RABC in this figure) exist primarily as monomeric active phosphatases, whereas the smaller extracellular domain of RO isoforms makes them more prone to dimerization. Homodimerization of RO reduces its activity and increases the signal transduction threshold (Hermiston et al. 2000)
Studies have demonstrated mutations in the gene PTPRC, which encodes CD4, and abnormalities in the expression of CD45 splice variants cause severe-combined immunodeficiency (SCID) in humans (Penninger et al. 2001). Autoimmunity, viral infections, few peropheral T cells and impaired T and B cells responses are also associated with abnormal CD45 splicing (Stanton et al. 2000). A polymorphism (C77G) in exon 4 that interferes with CD45 splicing causes activated or memory T cells to express both high-cD45RA and low-molecular-weight CD45RO isoforms, in contrast to the normal pattern of low-molecular-weight CD45RO isoform expression” (Stanton et al. 2000). Defective alternative splicing of CD45 has been further implicated in susceptibility to multiple sclerosis (MS), providing the first genetic evidence that alterations in CD45 splicing in humans can be associated with the development of MS in some families (Penninger et al. 2001).
Tolerance and long-term engraftment of allogeneic and xenogeneic heart and kidney transplants can be induced by the creation of anti-CD45RB antibodies that are reactive to defined CD45 epitopes (Penninger et al. 2001). Treatment with anti-CD45RB antibodies for a change in CD45 isoform expression on T cells, which in turn makes the T cell more conducive to allogeneic graft tolerance ( Fecteau et al. 2001)
Brains from a CD45-deficient transgenic mouse model of Alzheimer's disease exhibit an increase in tumor necrosis factor-alpha production compared to wild-type mice. Based on these findings, it has been proposed that the targeting of CD45 or its therapeutic modulation may be directly applicable to the treatment of autoimmune diseases or microglial activation associated with Alzheimer's disease (Penninger 2001). The modulation of CD45 splice variants may also allow for the treatment of cancer and autoimmunity by designing drugs that turn-off or turn-on antigen and cytokine receptor signaling (Penninger 2001).
Alexander, DR. (2000). The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Seminars in Immunology. 12:349-359.
Fecteau, S, Badasodonna GP, Freitas, A, Ariyan, C, Sayegh, MH, Rothestein, DM. (2001). CLTA-4 up-regulation plays a role in tolerance mediated by CD45. Nature Immunology. 2: 58-63.
Hayami-Noumi, K, T Tsuchiya, Y Moriyama, T Noumi. (2000). Intra- and intermolecular interactions of the catalytic domains of human CD45 protein tyrosine phosphatase. Federation of European Biochemical Societies. 468: 68-72.
Heath, WR. (2001). The secret if CD45. Trends in Immunology. 22: 123.
Hermiston, ML, X Zheng, A Weiss. (2003). CD45: A critical regulator of signaling thresholds in immune cells. Annual Review Immunology. 21: 107-37.
Janeway, A.C, P. Travers, M. Walport., J.M. Shlomchik.2005. Immunobiology: The Immune System in Health and Disease. 6th ed. New York, NY: Garland Publishing. pp 196, 416-417.
Johnson, KG, SK Bromley, ML Dustin, ML Thomas. (2000). A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proceedings of the National Academy of Sciences. 97: 10138-10143.
Leitenberg, D, Y Boutin, D Lu, K Bottomly. (1999). Biochemical Association of CD45 with the T Cell Receptor Complex: Regulation by CD45 Isoform and during T Cell Activation. Immunity. 10: 701-711.
Majeti, R, Z Xu, TG Parslow, JL Olson, DI Daikh, N Killeen, A Weiss. (2000). An inactivating point mutation in the inhibitory wedge of CD45 casues lymphoproliferation and autoimmunity. Cell. 103:1059-1070.
Ogimoto, M, Y Arimura, T Katagiri, K Mitomo, JR Woodgett, AR Nebreda, K Mizuno, H Yakura. (2001). Opposing regulation of B cell receptor-induced apoptosis of mitogen-activated protein kinases by CD45. FEBS Letters. 490: 97-101.
Penninger, JM, Irie-Sasaki1, J, Sasaki1, T, Oliveira-dos-Santos, AJ. (2001). CD45: new jobs for an old acquaintance. Nature Immunology. 2: 389-396.
Roach, T, S Slater, M Koval, L White, EC McFarland, M Okumura, M Thomas, E Brown. (1997). CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Current Biology. 7:408-417.
Stanton, T, S Boxall, K Hirai, R Dawes, S Tonks, T Yasui, Y Kanaoka, N Yuldasheva, O Ishiko, W Bodmer, PCL Beverley, EZ Tchilian. (2003). A high-frequency polymorphism in exon 6 of the CD45 tyrosine phosphatase gene (PTPRC) resulting in altered isoform expression. Proceedings of the National Academy of Sciences. 100: 5997-6002.
Tchilian, EZ and PCL Beverley. (2006). Altered CD45 expression and disease. Trends in Immunology. 27: 146-153.
Send comments, questions, and suggestions to: Rebecca Jameson
© Copyright 2006 Department of Biology, Davidson College, Davidson,
NC 28036