The lymphocyte protein-tyrosine kinase Lck (p56) is a member of the Src-family protein kinase family (Delves & Roitt, 1998). The LCK gene is on mouse chromosome 4 and is encoded by the 1p35-p32 segment in humans (Converse, 2003).
The Src-family kinases are covalently bound to the inner face of the plasma membrane by palmitoyl or myristoyl lipids and have several domains. The SH1 domain contains the active site, the SH2 domain can bind to phosphorylated tyrosine residues, and the SH3 domain interacts with proline-rich regions of other proteins (Janeway et al., 2005). One function of the Src-family kinases is to phosphorylate tyrosine residues on ITAMs (Delves & Roitt, 1998). Src-family kinases are regulated primarily by phosphorylation and by a negative feedback loop formed by the C-terminal Src kinase (Csk) and the transmembrane Csk-binding protein (Cbp). In regulatory phosphorylation, Src-family kinases are activated when the tyrosine residues at the kinase domain are phosphorylated and are inactivated when the tyrosine residues at the C-terminus are phosphorylated. In the negative feedback loop, Cbp gets phosphorylated and recruits Csk to the membrane. Subsequently, Csk inactivates Src-family kinases, including those that are phosphorylated at the kinase domain (Janeway et al., 2005). One additional regulator of Lck is the CD45 tyrosine kinase, which inhibits Lck activity in thymocytes. Current evidence suggests that CD45 inhibits Lck by dephosphorylating two tyrosine residues, Tyr 505 and Tyr 349. In the absence of CD45, cells have hyperphosphorylated tyrosine residues on Lck (D'Oro & Ashwell, 1999).
Lck associates with CD4, CD8, CD28, and the IL-2 receptor and is anchored to the cytoplasmic face of the plasma membrane through a myristylated amino-terminal glycine (Delves & Roitt, 1998). Currently, the most is known about the Lck association with the T cell co-receptors CD4 and CD8. Lck associates with the cytoplasmic domain of CD4; the negative amino acid residues at the amino-terminus of Lck and the basic residues in the conserved consensus sequence in the cytoplasmic domain of CD4 form ionic bonds (Delves & Roitt, 1998). Similarly, the amino-terminus of Lck associates with a highly conserved domain on the cytoplasmic tail of CD8α (Delves & Roitt, 1998; Janeway et al., 2005). The association of both co-receptors with Lck is zink-dependent (Kim et al., 2003). Although Lck is associated with both CD4 and CD8, the Lck kinase activity is higher when it is associated with CD4 (Delves & Roitt, 1998).

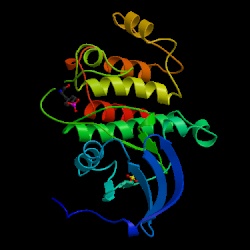
Figure 1: Kinse Domain of Lck. The activated kinase domain of human Lck is auto-phosphorylated on TYR394.
This image is courtesy of the Protein Data Bank. <http://www.rcsb.org/pdb/navbarsearch.do?newSearch=yes&isAuthorSearch=no&radioset=All&inputQuickSearch=Lck>
T cell development
Lck is involved in multiple aspects of T cell development including β chain rearrangement, differentiation into α/β or δ/γ T cells, and positive selection.
Briefly, the developmental stages of a thymocyte in the thymus are: 1) A stem cell 2) A double negative (DN) cell (CD4-CD8-) in which the β chain rearranges and, if successful, the cell will express the pre-TCR with the associated CD3 chains. At this stage, the cell expresses both the α/β pre-TCR and the δ/γ TCR; different signal(s) received will cause the cell to commit to either the α/β or δ/γ T cell lineage. 3) At the double positive (DP) cell stage (CD4+CD8+), the α chain will rearrange. If this rearrangement is successful, the cell will express a TCR. 3) The cell will undergo positive selection and become a single-positive T cell. 4) After completing negative selection, the mature, naïve T cell will exit the thymus.
Lck is first expressed in DN T cells and its expression continues into maturity. At the DN stage, Lck is required for successful β chain rearrangement and the subsequent expression of CD4 and CD8. Overexpression of Lck in developing thymocytes causes a decrease in Vβ -Dβ rearrangement "while permitting normal juxtaposition of other TCR gene segments" (Anderson et al., 1992). In mice with deficient Lck, developing T-cells do not reach the DP stage and the α chain does not rearrange. Also during the DN stage, immunologists believe that a Lck-mediated signal is involved in the lineage commitment to α/β or δ/γ T cells (Janeway et al., 2005). Saint-Ruf et al. (2000) have found evidence that supports this hypothesis. They have shown that the pre-TCR (α/β), but not the δ/γ TCR, colocalizes with Lck into glycolipid-enriched membrane domains without ligation. This phosphorylates and activates CD3ε and ZAP-70, which are involved in a subsequent signal cascade that may determine lineage commitment (Saint-Ruf et al., 2000).
Later in T cell development, positive selection occurs to generate MHC restriction. Positive selection involves the interaction of MHC with the antigen receptor (TCR) and co-receptors such that only cells with the correct co-receptor survive. While the exact mechanism of positive selection is currently unknown, it is believed that Lck-mediated signals from the co-receptor are involved in the cell's development into a CD4+ or CD8+ T cell (Janeway et al., 2005). The current model suggests that the double-positive thymocyte downregulates both CD4 and CD8. Next, it re-expresses CD4. At this time, there are two possible signals that the cell could receive: if the TCR and CD4 bind to MHC class II and Lck-mediated signaling is sustained, the cell will become a CD4+ T cell; if the TCR binds MHC class I, the Lck-mediated signal will be interrupted because there is no CD8 expressed and the cell will become a CD8+ T cell (Sarafova, 2006). The exact mechanism of positive selection and the role of Lck is currently under investigation.
T cell activation
A T cell is activated when the TCR and the co-receptor bind the antigen peptide:MHC complex, which causes the TCR complexes cluster and triggers an intracellular signal cascade. In T cell activation, the binding of the co-receptors CD4 or CD8 to MHC class II or class I, respectively, increases the sensitivity of T cells to antigen. The TCR complex includes the TCR (α and β chains), CD3 (δ, γ, and two ε chains), and two ζ chains (Janeway et al., 2005).
TCR signaling initially involves two Src-family kinases, Lck and Fyn. When antigen is recognized, the TCR complexes cluster. Clustering brings Lck, which is associated with the cytoplasmic domain of the co-receptor, to its targets in the TCR complex. Lck phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) on the cytoplasmic domains of the ζ and the CD3 δ, γ, and ε chains of the TCR complex. The SH2 domains of the protein-tyrosine kinase ZAP-70 subsequently bind to the phosphorylated ζ chain. Upon binding, ZAP-70 is activated and propagates the signal cascade. Ultimately, the signal cascade results in the activation of the transcription factors NFAT, AP-1, and NFκB in the nucleus and the T cell is activated (Janeway et al., 2005).
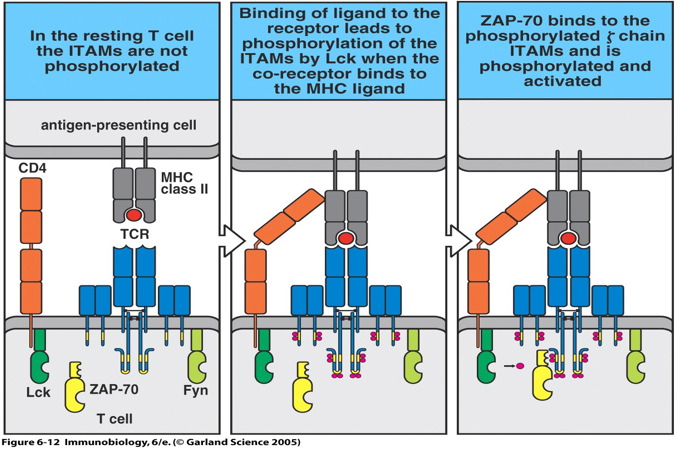
Figure 2: Activation of T cell signal cascade. The mature, naïve T cell is activated once the TCR and co-receptors bind to the peptide:MHC complex. The TCR complexes cluster allowing Lck to phosphorylate the ITAMs on the cytoplasmic domains of the TCR complex molecules. Subsequently, ZAP-70 binds to the phosphorylated ITAMs on the ζ chain and is activated to propagate the signal (Janeway et al., 2005).
Apoptosis
In recent years, immunologists have discovered and begun exploring the role of Lck in inducing apoptosis. However, the exact mechanisms of Lck involvement are still being investigated.
Lck is required for activation-induced T cell death (AICD) that is triggered if a circulating T cell binds to a partial agonist. In the AICD mechanism, the ligation of TCRs with partial agonists results in a partial TCR signal, mediated by Lck, that makes the cell sensitive to signals from death ligands such as the Fas ligand (Yu et al., 2004).
Lck is also necessary for initiating the mitochondrial apoptosis pathway (Samraj et al., 2006; Heyninck & Beyaert, 2005). In this pathway, the mitochondria ruptures, cytochrome c is released into the cytoplasm, and CAD (a caspase-activated DNase) is activated (Janeway et al. , 2005). Bcl-2 family proteins are the known regulators of B and T cell apoptosis (Samraj et al., 2006). It has recently been shown that Lck regulates expression of the Bcl-2 protein Bak independently of the Lck kinase activity (Heyninck & Beyaert, 2005). In Lck-deficient cells, Bak is not expressed (Samraj et al., 2006).
There are many diseases and conditions that involve T cell abnormalities including rheumatoid arthritis, asthma, organ transplantation, multiple sclerosis, inflammatory bowel diseases, type 1 diabetes, systemic lupus erythematosus, psoriasis (Kamens et al., 2001), Hereditary Haemochromatosis (Arosa et al., 1994), leukemia, Hodgkin lymphomas, neuroblasts, and others (Converse, 2003).
Research into some of these T cell-related conditions/diseases has not shown any connection between Lck and the pathogenesis of the disease. For instance, research to this date indicates that Lck abnormalities most likely do not play a major role in type 1 diabetes (Hulme et al., 2004). However, Lck impairments have been shown to contribute to the pathogenesis of other T cell-related diseases. Discovery of Lck-related diseases and conditions is important because drug treatments that inhibit Lck may improve the safety of treatment for T-cell-driven diseases because Lck expression is restricted to lymphoid cells (Kamens et al., 2001).
A number of human diseases are caused by abnormalities at the LCK locus (Converse; 2003). One example is T-cell acute lymphoblastic leukemia, which is caused by the translocation t(1;7)(p34;q34) with the TCRB gene (Converse, 2003; GeneCard, 2005). As a second example, LCK abnormalities are involved in the severe combined immunodeficiency (SCID) phenotype, which includes defects in cellular and humoral immunity. Mice either lacking Lck or expressing dominant-negative mutations in Lck showed SCID-like phenotypes that included T-cell developmental defects (Converse, 2003). Similarly, in an infant with severe SCID symptoms, selective CD lymphopenia, and lack of CD28 expression on CD8+ T cells, Lck was spliced such that it lacked the exon 7 kinase encoding domain (Goldman et al., 1998). A final example of a Lck-associated genetic condition is the autosomal recessive disease Hereditary Haemochromatosis (HH). The disease is associated with increased iron absorption and, in some cases, low numbers of CD8+ T cells in the periphery. HH patients also show a decrease in CD8-Lck specific activity that contributes to the pathogenesis and is not corrected by iron depletion (Arosa et al., 1994).
Other T-cell-associated diseases involve inhibition of Lck activity by various mechanisms. For instance, in Hodgkin's lymphoma and other tumors, there is decreased cellular immunity because of impaired CD4+ T cell activation. It has been suggested that the elevated levels of prostaglandin E(2) that are associated with Hodgkin's lymphoma inhibits CD4+ T cell function by inactivating Lck (Chemnitz et al., 2006).
Lck function is also impaired in people infected with the human immunodeficiency virus (HIV) and contributes to the pathogenesis of HIV, which is characterized by a depletion of CD4+ T cells. The HIV-encoded proteins Tat and Nef and the gp120 envelope gene product are involved in the associated T cell dysfunctions (Collette et al., 1996). The Nef protein of the human immunodeficiency virus type 1 (HIV-1) is required for the progression of HIV and for the maintenance of high viral loads; HIV strains that lack Nef do not progress to AIDS (Olszewski et al., 2004). Nef expression has been shown to cause depressed Lck kinase activity, which impaires Lck-mediated signaling events (Collette et al. , 1996). More recently, it has been shown that Nef triggers the internalization and degradation of CD4 primarily by disrupting the CD4-Lck complex (Olszewski et al., 2004). Specifically, a proline rich region in the N-terminal of the Nef protein binds to the SH3 domain of Lck to disrupt the CD4-Lck interaction (Briese et al., 2005). Currently, anti-HIV treatments are targeting a limited number of HIV proteins, so the development of new HIV drugs may increase treatment success. Olszewski et al. (2004) have shown that synthetic guanidine alkaloids of the batzelladine and crambescidin families can disrupt Nef-Lck interaction to inhibit HIV.
The role of Lck in inducing apoptosis has been utilized for cancer treatments and immunosuppressive drugs. Anticancer drugs and irradiation induce apoptosis of human lymphocytes and lymphoma cells via the mitochondrial apoptosis pathways. Gruber et al. (2004) found that Lck is essential for apoptosis induction by the drugs Doxorubicin, Paclitaxel, and 5-Fluorouracil. Lck is necessary for the early steps of the mitochondrial apoptosis signaling cascade, specifically the alteration of mitochondrial function and the activation of caspases (Gruber et al., 2004). Lck regulates these events by controlling the expression of the Bcl-2 protein Bak. Lck also plays a key role in drug resistance; T cells that are Lck deficient are resistant to anticancer drugs, but are still capable T cell death mediated by death receptors (Samraj et al., 2006).
A range of immunosuppressive drugs that induce apoptosis of lymphocytes can suppress harmful immune responses. Rosmerinic acid (RosA) is an immunosuppressive drug derived from herbal plants that induces apoptosis via the mitochondrial pathway. The effect of RosA is Lck-dependent, requiring the SH2 domain of Lck but not the Lck kinase activity, and independent of the Fas/Fas ligand interaction. Due to the requirement for Lck, RosA is expected to have selectivity towards Lck-containing cells, specifically T and NK (natural killer) cells. Thus, RosA may be a future treatment for T cell-mediated pathologic conditions such as rheumatoid arthritis, T cell leukemia, and transplant rejections. If RosA were proven to be an effective treatment for these conditions, it would be an improvement over drugs that are currently used for rheumatoid arthritis and transplant rejections because current drugs kill all leukocytes (T cells, macrophages, and monocytes). Also, because RosA apoptosis is independent of activation induced T cell death (ACID) and Fas/FasL interaction, RosA may be used in treating rheumatoid arthritis patients with AICD and Fas/FasL apoptosis-resistant T cells (Hur et al., 2004).
Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. 1992. Inhibition of T-cell receptor beta-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck [abstract]. In The EMBO Journal 11(13). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1334460&query_hl=48&itool=pubmed_docsum>. Accessed 2006 March 13.
Arosa FA, da Silva AJ, Godinho IM, ter Steege JC, Porto G, Rudd CE, de Sousa M. 1994. Decreased CD8-p56lck activity in peripheral blood T-lymphocytes from patients with hereditary haemochromatosis [abstract]. In The Scandinavian Journal of Immunology 39(5). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8191217&query_hl=9&itool=pubmed_docsum>. Accessed 2006 March 13.
Briese L, Preusser A, Willbold D. 2005. Mapping the binding site of full length HIV-1 Nef on human Lck SH3 by NMR spectroscopy [abstract]. In Journal of Biomedical Science 12(3). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15976924&query_hl=43&itool=pubmed_docsum>. Accessed 2006 March 12.
Chemnitz JM, Driesen J, Classen S, Riley JL, Debey S, Beyer M, Popov A, Zander T, Schultze JL. 2006. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck: implications in Hodgkin's lymphoma [abstract]. In Cancer Research 66(2). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16424048&query_hl=5&itool=pubmed_docsum>. Accessed 2006 March 12.
Collette Y, Dutartre H, Benziane A, Ramos-Morales, Benarous R, Harris M, Olive D. 1996. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. The Journal of Biological Chemistry 271(11). <ttp://www.jbc.org/cgi/content/full/271/11/6333>. Accessed 2006 March 13.
Converse PJ. 2003 Sept 24. Lymphocyte-specific protein-tyrosine kinase; Lck. Online Mendelian Inheritance in Man. <http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=153390>. Accessed 2006 March 9.
Delves PJ & Roitt IM, editors. 1998. Encyclopedia of Immunology Second Edition. San Diego: Academic Press.
D'Oro U & Ashwell JD. 1999. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. Journal of Immunology 162(4). <http://www.jimmunol.org/cgi/content/full/162/4/1879>. Accessed 2006 March 13.
GeneCard for protein-coding Lck GC01P032386. GeneCards. 2005 Oct 19. <http://www.genecards.org/cgi-bin/carddisp?LCK>. Accessed 2006 March 13.
Goldman FD, Ballas ZK, Schutte BC, Kemp J, Hollenback C, Noraz N, Taylor N. 1998. Defective expression of p56lck in an infant with severe combined immunodeficiency. The Journal of Clinical Investigation 102(2). <http://www.jci.org/cgi/content/full/102/2/421>. Accessed 2006 March 13.
Gruber C, Henkel M, Budach W, Belka C, Jendrossek V. 2004. Involvement of tyrosine kinase p56/Lck in apoptosis induction by anticancer drugs [abstract]. In Biochemical Pharmacology 67(10). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15130763&query_hl=15&itool=pubmed_docsum>. Accessed 2006 March 13.
Heyninck K & Beyaert R. 2005. A novel link between Lck, Bak expression and chemosensitivity [abstract]. In Oncogene [Epub ahead of print]. PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16186791&query_hl=20&itool=pubmed_docsum>. Accessed 2006 March 13.
Hulme JS, Barratt BJ, Twells RC, Cooper JD, Lowe CE, Howson JM, Lam AC, Smink LJ, Savage DA, Undlien DE, Guja C, Ionescu-Tiirgoviste C, Tuomilehto-Wolf E, Tuomilehto J, Todd JA. 2004. Association analysis of the lymphocyte-specific protein tyrosine kinase (LCK) gene in type 1 diabetes [abstract]. In Diabetes 53(9). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15331563&query_hl=49&itool=pubmed_docsum>. Accessed 2006 March 14.
Hur Y, Yun Y, Won J. 2004. Rosmarinic acid induces p56lck-dependent apoptosis in Jurkat and peripheral T cells via mitochondrial pathway independent from Fas/Fas Ligand interaction. The Journal of Immunology 172. <http://www.jimmunol.org/cgi/content/full/172/1/79>. Accessed 2006 March 12.
Janeway CA, Travers P, Walport M, Shlomchik MJ. 2005. ImmunoBiology, Sixth Edition. New York: Garland Science Taylor and Francis Group.
Kamens JS, Ratnofsky SE, Hirst GC. 2001. Lck inhibitors as a therapeutic approach to autoimmune disease and transplant rejection [abstract]. In Current Opinion in Investigational Drugs 2(9). PubMed database <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11717807&query_hl=7&itool=pubmed_docsum>. Accessed 2006 March 12.
Kim PW, Sun ZY, Blacklow SC, Wagner G, Eck MJ. 2003. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8 [abstract]. Science 301(5640). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14500983&query_hl=33&itool=pubmed_docsum>. Accessed 2006 March 12.
Olszewski A, Sato K, Aron ZD, Cohen F, Harris A, McDougall BR, Robinson WE Jr., Overman LE, Weiss GA. 2004. Guanidine alkaloid analogs as inhibitors of HIV-1 Nef interactions with p53, actin, and p56lck. Proceeding of the National Academy of Science of the United States of America 101(39). <http://www.pnas.org/cgi/content/full/101/39/14079>. Accessed 2006 March 12.
Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, Grassi F. 2000. Differentiation initiation of pre-TCR and gammadelta TCR signaling [abstract]. In Nature 406(6795). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10952314&query_hl=49&itool=pubmed_docsum>. Accessed 2006 March 13 .
Samraj AK, Stroh C, Fischer U, Schulze-Osthoff K. 2006. The tyrosine kinase Lck is a positive regulator of the mitochondrial apoptosis pathway by controlling Bak expression [abstract]. In Oncogene 25(2). PubMed database. <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16116473&query_hl=18&itool=pubmed_docsum>. Accessed 2006 March 13.
Sarafova, S. 2006 Feb 16. Immunology Lecture. Davidson College.
Yu XZ, Levin SD, Madrenas J, Anasetti C. 2004. Lck is required for activation-induced T cell death after TCR ligation with partial agonists. Journal of Immunology 172(3). <http://www.jimmunol.org/cgi/content/full/172/3/1437>. Accessed 2006 March 13.
Return to Immunology Main Page
E-mail questions & comments to: jaschwartz@davidson.edu