

Reporter genes are valuable tools in molecular biology because they allow researchers to visualize the protein products of genes. Green fluorescent protein (GFP) is unique because it naturally fluoresces with out the addition of substrates or enzymes, allowing researchers to view genetic expression in living cells. GFP can serve as a transcription marker or it can be used to visualize protein localization. The newly discovered red fluorescent protein (RFP) shares many properties of GFP and can be applied in a similar manner. One form of red fluorescent protein (DsRed), isolated from Discosoma striata and manufactured by Clontech, has an optimal absorption at 558 nm and emits light at 583 nm (CLONTECH, 1999). Another form of RFP (cob A) has been isolated from Propionibacterium freudenreichii (Wildt and Deuschle, 1999). One advantage of red fluorescent protein (RFP) is that it produces less background interference than GFP. Although the greatest advantage of RFP is that it can be used in conjunction with GFP to colabel cells.
Expression System
Cloning and Fusion
RFP is a reporter gene that allows
the visual detection of gene expression in living cells. This is
how it works. First the RFP gene must be fused to a gene of interest.
A commercially bought RFP gene is already inserted into a plasmid (there
are various types of plasmids
available that have been optimized for different types of research).
RFP gene plasmids (pRFP) have polylinkers located near the RFP gene.
The gene of interest and pRFP are cut with a restriction enzyme and mixed
together so they can ligate. Once the gene of interest is ligated
to the RFP plasmid, it can be cloned by inserting the pRFP into bacterial
cells. Depending on the plasmid being used, an ampR
gene
or another gene conferring resistance may also be found on pRFP.
Thus transformed bacteria can be selected by their resistance to a substance.
Again depending on the type of pRFP purchased, a promoter many need to
be inserted into the plasmid or the plasmid may already contain a promoter.
The plasmid can now be cloned via bacterial replication.
Expression
The vectors created in the previous
steps can be inserted into any living cells. Once inside, the chimeric
protein will be expressed through the natural process of transcription
and translation. This protein should contain a functional RFP and
protein of interest. A light of the appropriate wavelength must be shown
on the cells and the RFP will fluoresce, allowing visual detection of the
protein of interest.
Plasmids
available from Clontech
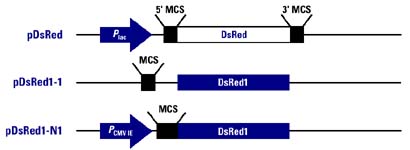
Figure 2. Plasmids containing the
RFP gene that are currently available from Clontech.
Image used with permission
from Clontech.
pDsRed: This is a cDNA copy of the RFP gene (DsRed). It is flanked by two polylinkers (MCS-multiple cloning sites). This plasmid also contains ampR and the lac promoter. Thus the RFP gene can be removed and inserted into a new plasmid or this plasmid can be inserted into the appropriate vector. This plasmid is optimal for nonmammalian expression.
pDsRed1-1: This plasmid contains an RFP gene that has been altered for human expression of RFP (DsRed1). A promoter must be inserted into this plasmid for RFP to be expressed. Rather than create a fusion protein, this vector is ideal for research on promoter and enhancer sequences in mammals.
pDsRed1-N1: This plasmid also
contains DsRed1 as well as a CMV promoter, making it ideal for creating
fusion proteins to be expressed in mammalian cells.
Microscopy
The expression of RFP can be visualized
using a fluorescence microscope with a rhodamine or FITC filter set (Wildt
and Deuschle, 1999). Colabelling is possible by transfecting cells
with different plasmids each containing one fluorescent protein gene and
one gene of interest (Figure 3). Double or even triple labeling a
cell is a highly informative tool. Now researchers can see the interaction
between gene products or the difference in localization between two or
more protiens. Another possible experiment is to monitor the progressional
expression of two or more genes, which would be very useful in developmental
studies.
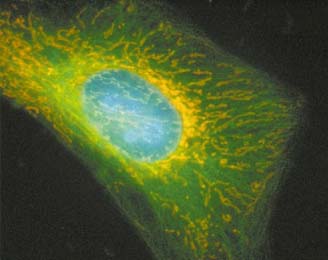
Figure 3. HeLa Cell transfected with three fusion fluorescent
proteins. Nuclear proteins
fused with enhanced cyan fluorescent
protein (ECFP). Tubulin proteins
labeled with EY(yellow) FP.
Mitochondrial proteins were labeled
with DsRed. Image used with
permission from Clontech.
Flow
Cytometry
Flow cytometry allows a mixed sample of cells to be sorted their physical
or chemical properties. Cells that are unusual in some way can be
collected for further research. Cells are labeled with a fluorescent
dye that can be excited by a beam of light. Cells that emit fluorescence
are detected and this information is stored for further study. In some
flow cytometry devices fluorescing cells are sequestered. DsRed serves
as such a dye. A 488nm argon laser can be used to excite DsRed in
flow cytometry experiments (CLONTECH, 1999).
All questions concerning the purchase
of this product should be directed to Clontech
Campbell, N. A. 1996. Biology, 4th ed. Menlo Park, CA: Benjamin/Cummings Publishing Company, Inc., . p. 372-374.
CLONTECH Laboratories. 1999. Living Colors Red Fluorescent Protein.
CLONTECHniques.October:2-6.
<http://www.clontech.com> accessed
2000 13 Feb.
The John Curtin School of Medical Research. 1998 March 12.
JCSMR Flow Cytometry "An introduction".
<http://jcsmr.anu.edu.au/facslab/intro.html>.Accessed
2000 20 Feb.
Wildt, S and Deuschle, U. 1999. cobA, a red fluorescent transcriptional
reporter for Escherichia
coli, yeast, and mammalian cells. Nature Biotechnology.
17: 1175-1178.
Return To Davidson College Biology Department Home Page
Return To Biology Course Materials