The ecdysone-inducible mammalian
expression system is a new technique offered by Invitrogen
to control gene expression. The technique offers quick, efficient
cloning of PCR products, and once cloned the gene product can be easily
expressed, detected and purified. This expression system is founded
by a mechanism utilized by molting insects. Scientist recognized
that molting was triggered by the steroid, ecdysone. Ecdysone is
an inducer steroid that binds to a receptor that stimulates the expression
of a target gene (1). Adapting this mechanism to cloned genes allowed
gene expression to be controlled and amplified by induction.

Figure 1 was provided by http://www.Invitrogen.com/manuals.html.
The ecdysone control mechanism utilizes
a transcriptional activator which is triggered by a steroid similar to
ecdysone. The mechanism consists of an inducible receptor in one
vector plasmid and two subunits and the cloned gene in another plasmid
vector. When these two vectors are cotransfected into a cell, the
introduction of an inducer allows transcription to begin. The inducer
actually binds to the receptor causing the two subunits to interact.
This combination gives the gene the right conformation and transcription
of the cloned gene begins. The ecdysone system is tightly regulated to
express the cloned gene because the receptor is specific for one of two
mammalian regulatory steroids; ponasterone A or muristerone A. This prevents
non-specific induction. To increase efficiency, five copies of the
receptor are upstream in the vector containing the cloned gene. These
control mechanisms provide for an undetectable base line expression rate
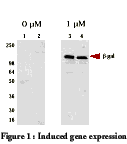
which when induced can be increased 200 fold (see figure 1).
The lanes in figure 1 represent cells transfected with a
cloned gene which were either uninduced (lanes 1 and 2), induced with muristerone
A (lane 3), or induced with ponasteroneA (lane 4). Then the lanes
were probed with an antibody to determine the relative amounts of protein
produced by each condition. Clearly, the induced cells were effected
by the inducer, and therefore produced the protein from the cloned gene
of interest (2).
This technique has created a tight
control mechanism to express the gene, but first the gene needs to be cloned.
Invitrogen utilizes a system called topo cloning to efficiently clone a
gene. Topo cloning offers a new way to quickly clone taq-amplified
PCR fragments. This technique utilizes topoisomerase 1 rather than
ligase to ligate PCR products to a vector. This method is more efficient
than other PCR cloning procedure because it lacks ligases, special primers,
and modifications and clean-up of the PCR fragments. The cloning
takes about five minutes and provides a high level of efficiency.
Most PCR fragments can be cloned with an efficiency of 95% recombinants
of the gene fragment into the plasmid vector (3).
Finally, Invitrogen offers a
broad range of vector characteristics to use along with these new techniques
to increase the vectors usability for many experiments. The major
advantages that they have added to their vectors include; multiple cloning
sites, a choice of resistance genes, and an epitope and tag. Most
of the vectors contain 15 or more restriction sites into a reporter gene
which makes cloning much simpler. The different resistance genes
allows a researcher to quickly select stable cell lines that include a
cloned fragment. The epitpe and tag help to ease detection and purification
of your gene. These techniques and products offer a high level of
control, flexibility, and efficiency to make cloning and gene expression
quicker and easier than ever before (3).
1 Campbell, N. Biology . California: Benjamin/ Cummings, 1996.
2 "Precise Control of Inducible expression in Mammalian Cells." Ecdysone
Inducible System.
<http://www.Invitrogen.com/manuals.html>
Accessed 1999 Feb 7.
3 "The Fastest Way to Clone PCR products." Topo-Cloning .
<http://www.invitrogen.com/manuals.html>
Accessed 1999 Feb. 7.
Send comments, and suggestions to: Mocamp@davidson.edu