This web page was produced as an undergraduate assignment for Davidson College.
Proteomics
of FAR1 and YBR293W
|
This assignment was designed to explore
the technology available to better understand the proteome of Saccharomyces
cerevisiae. The proteome is all
of the proteins present in a cell at one point in time. When sudying proteomics one wants to
determine protein expression, protein location, protein amount, protein function, and protein interactions
for each protein. Proteomics aids in
understanding translational output. Specifically,
this assignment examines the role of the protein products of FAR1 and YBR293W
in the yeast proteome. Both of these
ORFs have been studied previously in Assignment
2- My Favorite Yeast Genes: FAR1 and YBR293W and Assignment
3- DNA Microarray Data for FAR1 and YBR293W. |
|
FAR1 |
|
This
annotated gene encodes a cyclin-dependent protein kinase inhibitor involved
in cell cycle arrest. |
TRIPLES Database (Transposon-Insertion Phenotypes, Localization, and Expression in Saccharomyces)
No records were available for FAR1.
Database of Interacting Proteins (DIP)
This is David Eisenberg’s database on protein-protein interactions. FAR1p has ten known interactions on this database. FAR1p is a cell cycle arrest protein that inhibits cyclin-dependent protein kinase. The interactions shown below confirm my preliminary research. BEM1 is also involved in the protein-kinase cascade that arrests the cell cycle at the G1 phase (Lyons 1996), so this result is consistent as well.
Table 1-1. Protein
interactions with FAR1p. This table
illustrates ten protein interactions with FAR1p using DIP. Original figure can be found here: http://dip.doe-mbi.ucla.edu/dip/DIP_Browse_nongraphical.cgi?PKEY=2229.
|
CENTERED ON |
|||||
|
Links |
Interaction |
PIR |
SWISSPROT |
GENBANK |
NAME |
|
- |
S56940 |
FAR1_YEAST |
1077109 |
factor arrest protein FAR1 |
|
|
INTERACTS WITH 10
PROTEINS |
|||||
|
S23400 |
BEM1_YEAST |
82912 |
bud emergence mediator BEM1 |
||
|
TVBY8 |
CC28_YEAST |
66745 |
protein kinase cdc28 |
||
|
S51452 |
CC42_YEAST |
1071931 |
cell division control protein CDC42 |
||
|
COBYC2 |
CG12_YEAST |
69009 |
cyclin 2 |
||
|
A27477 |
CC24_YEAST |
83456 |
cell division control protein CDC24 |
||
|
COBYC1 |
CG11_YEAST |
1070534 |
cyclin 1 |
||
|
S14054 |
CG13_YEAST |
83610 |
CLN3 protein |
||
|
S60939 |
GBB_YEAST |
2133158 |
GTP-binding protein beta chain STE4 |
||
|
S45760 |
LSM2_YEAST |
626847 |
probable snRNP-related protein YBL026w |
||
|
S50443 |
YEB6_YEAST |
1077597 |
hypothetical protein YEL016c |
||
Yeast Resource Center
Two-Hybrid Analysis
This database was produced from Stan Fields work with the yeast two-hybrid system in which a bait protein is used to find what prey proteins physically interact with it.
Table 1-2. FAR1p interactions using Y2H method. FAR1 is the prey protein when both BEM1 and CDC24 proteins are used as bait. Original figure can be found here: http://depts.washington.edu/%7Eyeastrc/th_12.htm.
|
Bait |
Prey |
Prey ORF |
|
BEM1 |
FAR1 |
YJL157C |
|
CDC24 |
FAR1 |
YJL157C |
This database reports protein-protein interactions. The follwing are proteins that interact with FAR1p: Bem1p [E]; Cdc28p+Cln1p [E]; Cdc28p+Cln2p [E]; Cdc28p+Cln3p [E]; Cdc24p [E]; Cdc42p [E]; Ste4p [E]. These interactions were already known in assingment 2 and are part of the protein-kinase cascade.
Schwikowski
and Uetz PDF
FAR1 is near both BEM1 and CDC24 as expected since its protein product is known to interact with these two proteins. SLA2 and RSR1 were also located near FAR1 on this figure. SLA2 is a gene that produces a transmembrane protein involved with membrane cytoskeletal assembly, cell polarization, and endocytosis (SGD 2001, http://genome-www4.stanford.edu/cgi-bin/SGD/search/quickSearch?query=SLA2). This file indicates that SLA2 is involved with vesicular transport. FAR1 has also been implicated in cell polarization (Butty et al. 1511), so this fits with the previous research. RSR1 is a gene that is invovled in bud site selection (SGD 2001, http://genome-www4.stanford.edu/cgi-bin/SGD/search/quickSearch?query=RSR1). I am not sure if these two proteins truly interact.
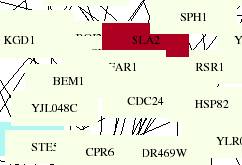
Figure 1-1. Aging PDF
zoomed in 900%. This figure shows FAR1
and its protein interactions. Indian
red represents genes whose protein products are involved with
vesicular-transport.
This file illustrates the same relationships as the aging
file. This figure indicates that FAR1
is involved in the mating response—a known fact from assignment 2 since
transcriptioin of FAR1 is induced by the secretion of a mating pheromone. CDC24 and STE5 are also indicated to be
involved with the mating response as they are in the proetin-kinase cascade
that ensues after the secretion of the mating pheromone (Peter and Herskowitz
1230). Here SLA2 is involved in cell
wall maintenance, which follows what was learned previously.

Figure 1-2. Degradation
PDF file zoomed in 900%. This figure
shows FAR1 and its protein interactions.
Orange-red represents those genes whose proteins are involved with
mating response and light blue represents those involved with cell wall
maintenance.
This file illustrates the same interactions as the aging file. FAR1 is involved with RNA turnover in this figure and BEM1 is involved in small molecule transport.

Figure 1-3. Degradation
PDF file zoomed in 900%. This figure
shows FAR1 and its protein interactions.
Indian red represents those genes whose proteins are involved with small
molecule transport and black represents those involved with RNA turnover.
This file illustrates the relationships of FAR1p with other proteins. FAR1 is shown to interact with BEM1, SLA2, and CDC24. However, no interaction is seen with RSR1, which confirms my conjecture that the two proteins do not actually interact directly.
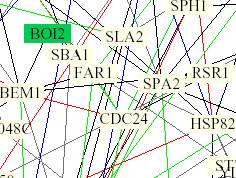
Figure 1-4. NB Figure1
PDF zoomed in 1200%. This figure shows
FAR1 and its protein interactions.
Green lines indicate that the cellular roles are identical but
localizations are different. Black
lines indicate that the cellular role and localization are different or unkown.
Kinase
sequence phylogenetic tree PDF
This file was generated using kinase phosphorylation yeast chip data. FAR1 was not found in this file because it is not a kinase, rather it inhibits kinase.
This database did not have any protein information
regarding FAR1.
Enzymes and Metabolic
Pathways Database
This database did not contain any protein information regarding FAR1.
|
YBR293W |
This unannotated gene (hypothetical
ORF) has an unknown biological function.
After the initial sequence analysis, I proposed that YBR293W was
involved with budding. The DNA
microarray analysis led me to believe that this unannotated gene product was
included in a signal transduction pathway.
TRIPLES Database (Transposon-Insertion Phenotypes, Localization, and Expression in Saccharomyces)
No records were available for YBR293W.
Database of Interacting Proteins (DIP)
This is David Eisenberg’s database on protein-protein interactions. YBR293W had one protein interaction with JSN1, a benomyl dependent tubulin mutant (SGD 2001, http://genome-www4.stanford.edu/cgi-bin/SGD/search/quickSearch?query=jsn1). Two interesting pieces of information were gathered from these results: YBR293W is a probable resistance gene and is a transmembrane protein. I conjectured that this unannotated ORF was an integral membrane protein in assignment 2 when using a Kyte-Doolittle Analysis, and this database confirmed my suspicions.
Table 2-1. Protein
interaction with YBR293W. This table
illustrates the interaction between YBR293Wp and JSN1p using DIP. Original figure can be found here: http://dip.doe-mbi.ucla.edu/dip/DIP_Browse_nongraphical.cgi?PKEY=5142.
|
CENTERED ON |
|||||
|
Links |
Interaction |
PIR |
SWISSPROT |
GENBANK |
NAME |
|
- |
S46175 |
YB8G_YEAST |
626840 |
probable resistance protein YBR293w |
|
|
INTERACTS WITH 1
PROTEINS |
|||||
|
S57112 |
JSN1_YEAST |
1077899 |
JSN1 protein |
||
Yeast Resource Center
Two-Hybrid Analysis
This database was produced from Stan Fields work with the yeast two-hybrid system in which a bait protein is used to find what prey proteins physically interact with it. YBR293W was neither identified as a bait protein nor as a prey protein.
This database reports protein-protein interactions. The follwing protein interacts with YBR293W: Jsn1p [E] [details]. This interaction was already determined using the Database of Interacting Proteins.
Schwikowski
and Uetz PDF
YBR293W was not found on any of these files.
Kinase
sequence phylogenetic tree PDF
This file was generated using kinase phosphorylation yeast chip data. YBR293W was not found in this file because it was not a known kinase tested in the microwell array experiments of Mike Snyder.
This database did not have any protein information
regarding YBR293W.
Enzymes and Metabolic
Pathways Database
This database did not contain any protein information
regarding YBR293W.
So far, I know that JSN1 interacts
with this unannotated ORF. JSN1
suppresses tub2-150 and tub2-404, which are defective in spindle elongation,
when it is overproduced by increasing sensitivity to benomyl. JSN1’s cellular role is RNA turnover and
cell structure. It’s biochemical
function is an RNA-binding protein (YPD 2001, http://www.proteome.com/databases/YPD/reports/JSN1.html). I also know that YBR293W is a probable
resistance gene according to the Database of Interacting Proteins. In the DNA microarray analysis from
assignment 3, YBR293W clustered with transport (PMC1 and SSY1), stress response
(NTH1 and SSA3), and signal transduction (GPA1 and CDC37) genes twice out of
seven DNA microarrays. I proposed that
YBR293W was related to mating signalling and part of a responsive pathway that
functions to protect the yeast in some capacity. In assignment 2, I proposed that this ORF was involved with
budding. However, this ORF only
clustered with a transcription initiation factor or a gene involved in meiosis
one time. But, this ORF was induced and
then repressed during the sporulation experiment, so a function with budding was
still a possibility. This ORF was
probably not involved in respiration or metabolism though since its expression
was not affected by those experiments.
Experiment
1
In order to determine how this protein functions, I would first resolve what proteins interacted with YBR293W. To accomplish this feat, I would use a yeast two-hybrid analysis. YBR293W would be included in the bait protein along with a DNA binding domain. For prey, I would test JSN1 as a control (I know that there is an interaction) and all proteins involved with transport, stress response, signal transduction, mating signalling, budding, and resistance coupled with an complementary activation domain. His3 would be used as the reporter gene. The yeast would be grown on a His3(-) medium in order to determine what prey interact with the bait. As an additional control, I would grow the bait and the prey separately in order to ensure that neither ORF was the His3 gene.
I would predict that YBR293W would likely interact with yeast proteins from each group, especially the budding proteins since JSN1 seems to be involved cell structure.
|
References |
Butty AC, et al.1998.
The Role of Far1p in linking the heterotrimeric G protein to polarity
establishment proteins during yeast
mating. Science
282:1511-1516.
Database of Interacting Proteins. 2001. <http://dip.doe-mbi.ucla.edu/> Accessed 8 Nov 2001.
EMP Database. 2001. <http://emp.mcs.anl.gov/cgi-bin/map_search.pl> Accessed 8 Nov 2001.
Kumar A et al. 2000. TRIPLES: a Database of Gene
Function in S. cerevisiae. Nucleic Acids Res 28: 81-84.
Lyons DM et al.
1996. The SH3-domain protein Bem1 coordinates mitogen-activated protein kinase
cascade activation with cell
cycle control in Saccharomyces
cerevisiae. Mol Cell Biol 16: 4095-4106.
Peter M, Herskowitz I.
1994. Direct Inhibition of the Yeast Cyclin-Dependent Kinase Cdc28-Cln by Far1.
Science 265:1228-1231.
Ross-Macdonald
P et al. 1999. Large-scale analysis of the yeast genome by transposon
tagging and gene disruption. Nature
402, 413-418.
Schwikowski B, Uetz P, and Fields S. 2000. A network of protein-protein interactions in yeast. Nature Biotechnology 18: 1257-1261.
Uetz P et
al. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:
623-627.
WIT Database. 2001. <http://wit.mcs.anl.gov/WIT2/CGI/index.cgi>
Accessed 8 Nov 2001.
Zhu H et al. 2000. Analysis of
yeast protein kinases using protein chips. Nature Genetics. <http://www.nature.com/cgi-taf/DynaPage.taf?file=/ng/journal/v26/n3/full/ng1100_283.html>
Accessed Nov 8 2001.