Analysis
of Black Sea Turtle (Chelonia mydas
agassizii) population age structure, growth rate, and mortality composition
in Bahía Magdalena, Mexico
Gray Lyons, Davidson College
Lauren Bonenberger, Bates College
Lauren Daly, St. Michael’s College
School
for Field Studies
Center
for Coastal Studies
Baja
California Sur, Mexico
Advisor:
Volker Koch, Ph.D.
6
December 2002
Abstract
Bahía
Magdalena, Baja California Sur, Mexico is an important habitat for several
species of endangered sea turtles. Black
Sea turtles (Chelonia mydas agassizii) depend on the area as a nursery and
feeding ground. In the past
century, large-scale commercial fishing pressure and small-scale harvesting by
local peoples have severely reduced the Black turtle populations.
During this study, we investigated size distribution, growth rates,
species composition, and minimum mortality rates for sea turtles in the region.
Significance tests confirmed that larger Black turtles were found closer
to the open bay and smaller turtles were found further up Estero Banderitas, an
estuary in Bahía Magdalena. Data
from recaptured turtles was used to model a growth curve described by the
equation Lt
= 100*[1-e{-0.05*t-0.65*0.05/2π*(t-0.72)}]. Using this equation we were able to determine the approximate
age of a Black turtle by its straight carapace length (SCL). The pecentage of
Black turtle mortality decreased steadily from 2000 to 2002.
Different locations in Bahía Magdalena had different levels of Black
turtle mortality in that time period. The
majority of Black turtle carapaces found were juveniles and trend analysis
showed that the average SCL has been decreasing since 1995.
This study was conducted to contribute to the understanding of sea turtle
populations in Bahía Magdalena and to promote turtle conservation in the area.
Resumen
Bahía
Magdalena, Baja California Sur, México es un hábitat importante para varias
especies de tortugas marinas en peligro de extinción.
La tortuga Negra depende especialmente de esta área ya que la utiliza
como lugar de crecimiento y alimentación.
En el ultimo siglo, la presión de pesca comercial a gran escala y la
cosecha por gente local a menor escala redujeron gravemente la población
de tortugas Negras. Durante este
estudio, nosotros investigamos la distribución por tamaños, tablas de
crecimiento y niveles mínimos de muerte de tortugas marinas en esta región.
Nuestro estudio confirmo que las grandes tortugas Negras se encuentran próximas a mar abierto y las
tortugas mas pequeñas se encuentran mas cercanas a Estero Banderitas . El tamaño frecuencia distribución marcó una
diferencia
significativo con las tortugas grandes cerca de la bahía abierto.
Pruebas significo, captura para una ves mas a hacer un modelo de
desarrollo, Lt = 100*[1-e{-0.05*t-0.65*0.05/2π*(t-0.72)}].
Usando este modelo, nosotros determinamos la edad aproximada de una tortuga
Negra a través del tamaño del caparazón (SCL).
El porcentaje de tortugas Negras muertas descendió entre los años 2000 y 2002.
Lugares diferentes en Bahía Magdalena tienen niveles diferentes de
mortalidad de tortugas Negras en esta época.
La mayoría de carapachos de tortugas negras son juveniles y analices
mostraron que el SCL esta bajando desde 1995.
Este estudio fue llevado a cabo para contribuir al conocimiento de las poblaciones
de tortugas marinas en Bahía Magdalena y para promover la conservación de
tortugas en este región.
Introduction
Five
species of sea turtles occur on the Pacific side of Mexico, the Eastern Pacific
Green Turtle (Chelonia mydas), the
Loggerhead (Caretta caretta), the
Hawksbill (Eretmochelys imbricata),
the Olive Ridley (Lepidochelys olivace),
and the Leatherback, (Dermochelys coriacea),
all of which are endangered.
In
Mexico, turtles have been caught traditionally for consumption, leather, oil,
carapaces, and various other uses. In
the past century sea turtles were exploited by large-scale industries causing a
drastic decrease in population (Clifton et al., 1995).
In 1990, the Mexican government moved to stop the population decline by
implementing a complete ban on the extraction, capture, and pursuit of sea
turtles (DOF, 1990). Sea turtle harvesting continues despite the current legal
framework. Lack of enforcement of
this ban and a long-standing cultural tradition of sea turtle consumption
inhibit the conservation of sea turtles (Gomez, 1995).
Sea
turtles are also unintentionally caught as bycatch in gill nets and trawls not
equipped with turtle exclusion devices (TEDs).
Some fishermen refuse to use TEDs, claiming that they reduce the catch of
target species (Rudloe, 1989). Sea
turtles are also threatened by pollution, nesting beach destruction, and habitat
degradation (Alvarado-Diaz et al., 2001).
The
Eastern Pacific Green Turtle (Chelonia
mydas), known locally as the Black turtle (Chelonia mydas agassizii), is the most common species of sea turtle
found in the Bahía Magdalena region (Garcia-Martinez and Nichols, 2000).
Black sea turtles take at least twenty years to reach sexual maturity,
the last fifteen of which are spent in shallow bay and lagoon nursery grounds,
such as Bahía Magdalena. These nursery grounds often leave juvenile turtles vulnerable
to humans (Marquez, 1990). Many sea
turtles are harvested before they become sexually mature.
Studies estimate that the current Black turtle population is less than 2%
of its original size which could indicate a high fishing stress on the juvenile
population endangering the entire species (Nichols2001).
Because the turtle life cycle is slow, recovery from drastic population
declines is difficult and requires several decades of stringent conservation.
The
overall objective of the project is to aid in the conservation of sea turtles. There are three specific objectives of this study as part of
an ongoing investigation in Baja California.
First, we will establish the population size structure in several areas
of Bahía Magdalena to determine habitat preferences of different age classes of
Black turtles. Second, we will to
determine individual growth rates to create a model correlating turtle size and
age. Third, we will estimate the
sea turtle mortality rate, size, and composition in the region to isolate areas
for further study and conservation. Data
from this investigation will contribute to the understanding of sea turtle
ecology and promote sea turtle conservation.
Possible
turtle conservation projects include development of ecotourism, expansion of
environmental education, and the protection of habitats.
Establishing a marine protected area (MPA) in Estero Banderitas, a
juvenile nursery in Bahía Magdalena, would restrict the types of fishing
activities and increase patrolling in the estuary, thereby strengthening the
enforcement of pre-existing conservation laws.
If this feeding ground and nursery area is protected, Black sea turtles
could develop sufficiently for migration, breeding, and reproduction (Miller
1997).
Materials
and Methods
Live
Turtles
This
study was conducted at varying locations along Estero Banderitas in Bahía
Magdalena, Baja California Sur, Mexico (appendix
A).
Two standard cotton turtle entanglement
nets with lengths of 120 and 140 meters and depths of 7 meters were used.
The nets used had buoy lines along the top and light lead lines along the
bottom. The lighter weights are
important because as turtles become entangled in the nets they need to be able
to lift the net to the surface to breathe.
Starting at low tide, the nets were set across two channels in Estero
Banderitas and checked for turtles every 1.5 hours for about 12 hours.
Any turtle caught was placed in the panga for later identification and
measurements. GPS and temperature
measurements were recorded at the time of the first and last net check. Information for each turtle was recorded on Wildcoast Sea
Turtle Information Forms (appendix
B).
The
following data was recorded for each specimen: date, time, and location of the
capture. The following measurements
were taken using large calipers: straight carapace length (SCL), straight
carapace width (SCW), body depth, plastron length, and head width.
The following measurements were taken using measuring tape: curved
carapace length (CCL) and curved carapace width (CCW) (appendix
D). The
following measurements were taken using small calipers: total tail length (end
of plastron to tip of tail) and partial tail length (cloaca to tip of tail).
The turtles were weighed using a spring balance. The number of central,
left and right lateral, and left and right marginal scutes were counted.
The coloration of the carapace and plastron were evaluated and recorded
qualitatively. The species,
location, and number of any epibionts were recorded.
New turtles were given metal tags on both rear flippers and previous tag
numbers of recaptures were recorded for identification.
Turtles
caught for the first time were photographed and assigned an identification
number based on location of capture (BMA = Bahía Magdalena), the date (day,
month, year) and daily capture number (sequential). A skin sample was taken from
the neck and preserved in dimethyl sulfoxide (DMSO) for future genetic analysis.
The
growth rates of recaptured turtles were calculated using SCL measurements. The
previous SCL was compared to the recapture length.
The original measurement was then subtracted from the recapture, divided
by the numbers of days at large, and then multiplied by 30 to get the number of
centimeters per month. The von Bertalanffy growth function was used to determine
the age of the turtle (Chaloupka and Musick, 1997).
Turtle
Mortality
Over the course of three years, dumps and beaches in the Bahía Magdalena region were scoured for turtle carapaces and other remains. Each location was investigated between one and five times a year. Each carapace was measured for SCL, categorized by species, condition, and final disposition (appendix C). Carapaces were then transported to a turtle graveyard or marked with spray paint to prevent recounting. Turtle mortality data did not include plastrons, only carapaces were counted. Turtles were catalogued as “consumed” or “not consumed” depending on their condition. Examples of “consumed” turtles include charred carapaces and carapaces with harpoon holes, whereas examples of “not consumed” turtles include decomposing turtles washed up on a beach or whole turtles delivered dead on arrival. Note that “not consumed” turtles include mortality by anthropogenic factors (trawlling nets and long-line fishing) as well as natural factors. Locations visited include those marked with a star on the enclosed map (appendix A). Some data taken from Lopez Mateos measured CCL instead of SCL. In order to convert CCL to SCL the following equation was constructed from live Black turtle measurements: SCL=0.83*CCL+6.6, (n=213, R2=0.98).
Results
Live
Turtles
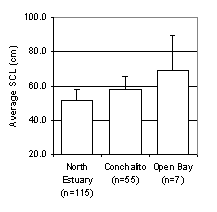
The average size of live sea turtle captures varied among locations in Bahía Magdalena. Figure 1 is a size frequency distribution indicating that on average, the open bay contains larger turtles than locations further up Estero Banderitas (Fig. 1). One-way ANOVA by Tukey’s post-hoc comparison (n=181) indicates a significant correlation between location and SCL (p<0.001).

Figure
1. Average straight carapace length
of Black turtle captures at varying locations in Estero Banderitas (appendix
A).
Error bars represent one standard deviation; n-values indicate the total
number of turtles caught per location from 2000 to 2002.
The growth rate of Black sea turtles was calculated from 52 recaptured Black turtles, ranging in SCL from 43.0 cm to 72.3 cm. Assuming a theoretical maximum SCL (Linf) of 100 cm, the growth curve derived from the Gulland and Holt plot shows the relationship between SCL and age for turtles sampled (Fig. 2). This relationship can be used to estimate the age of a turtle from its SCL.
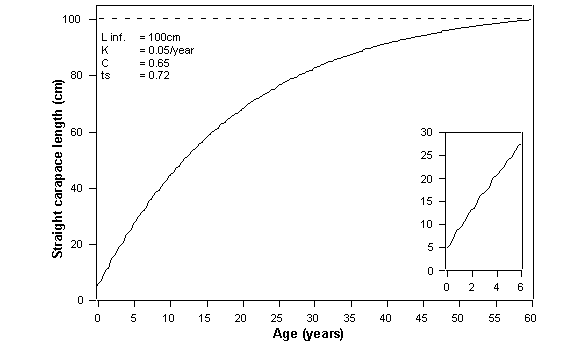
Figure
2. Graph on von Bertalanffy growth
function, obtained with the Gulland and Holt plot of recaptured turtle data
(n=52, range 43.0 cm to 72.3 cm). The graph is described by the following
equation: Lt = 100*[1-e{-0.05*t-0.65*0.05/2π*(t-0.72)}]
Though size at maturity varies between individual turtles, for our purposes we will draw on the estimates of Nichols (2001) and Marquez (1990) and consider Black turtles longer than 75 cm to be sexually mature. In three years of sampling, less than 3% of those captured have been larger than the 75 cm maturity mark. The size histogram below illustrates the large number of juvenile turtles captured (Fig. 3). Approximately 70% of the turtles captured were between 45 and 60 cm long.
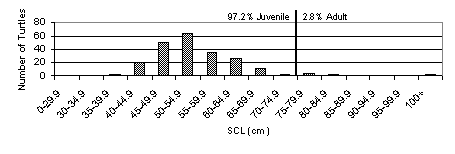
Figure 3.
Size histogram of live Black sea turtle captures grouped by size class
and separated by maturity.
Of the dead turtles occurring in Bahía Magdalena, Black and Loggerhead turtles have historically been found more frequently than other species (Fig. 4). Although more than half of all carapaces found in 2000 were Black turtles, Loggerheads were the more dominant species found in 2001 and 2002. The level of Black turtle mortality dropped 16% from 2000 to 2002.
Figure
4. Species composition of dead turtles by year; n-values
indicate the minimum sea turtle mortality per year.
The turtle mortality data from different towns and beaches in Bahía Magdalena varied in species composition (Fig. 5). At five of eight survey areas, Puerto San Carlos, Bahía Santa Maria, Puerto Chalé, Puerto Alcatraz, and Puerto Magdalena, the Black turtle was the dominant species found. At Lopez Mateos, Lopez Mateos Beach, and Santo Domingo, the Loggerhead was the most frequently occurring species.
Figure 5. Species composition of dead turtles found at various locations in Bahía Magdalena. N-values indicate total number of dead turtles found between year 2000 and 2002.
From 2000 to 2002, the percentage of minimum mortality due to consumption remained relatively constant between 77.4% and 81.0%. While the total number of dead turtles found has increased from 2000 to 2002, the number found in the largest town in the region, Puerto San Carlos, has been decreasing. Figure 6 shows a visible trend towards less turtle consumption in this town.
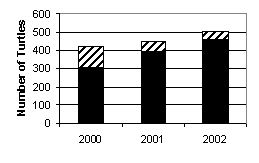
Figure
6. Yearly turtle mortality in
Puerto San Carlos (striped regions) as a fraction of total mortality (striped
and black regions) in Bahía Magdalena.
Before 2002, the average SCL of the Black turtle had been decreasing steadily. Though the average SCL for 2002 did increase slightly from 2001, the average SCL has decreased 13% since 1995 (Fig. 7). Figure 8 represents the SCL of each individual carapace found throughout the study period. The negative slope of the regression line of all Black turtle carapaces found indicates that the SCL of Black turtles is decreasing.
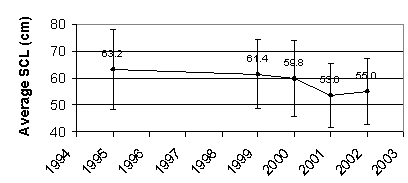
Figure
7. Average straight carapace length of dead turtles.
Error bars represent one standard deviation.
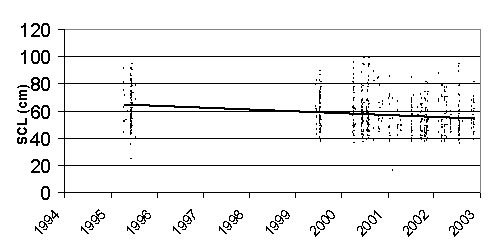
Figure
8. Scatter plot and regression line
of all Black sea turtle mortality findings.
The size frequency distribution below indicates that the majority of Black turtle carapaces found were juveniles (Fig. 9).
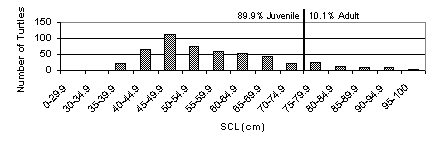
Figure 9. Size histogram of Black sea turtle mortality grouped by size class and separated by maturity.
Discussion
Live
Turtles
This study indicates that a size gradient exists within Bahía Magdalena with the smallest Black turtles further up Estero Banderitas and the larger ones closer to the open bay (Fig. 1). Since smaller turtles are more often found in the upper estuary, it is necessary for conservation efforts to include remote locations in the estuary to protect the immature individuals. Further study is required to validate the current data, especially in estimating the average turtle size in the open bay.
Figure
2 shows the relatively slow growth rates of Black sea turtles, while figure 3
shows the overwhelming percentage of juveniles in the bay.
Data suggests that Black sea turtles enter the bay measuring 35 to 45 cm,
and the majority (approximately 70%) of the Black turtles captured measured 45
to 60 cm (Fig. 3). Previous research also indicates that sea turtles
approximately 75 cm and over are sexually mature (Nichols, 2001; Marquez, 1990).
Using the growth equation (Fig. 2) we estimate that sea turtles enter the
bay at 7 to 10 years old, the majority of sea turtles captured are 10 to 15
years old, and sea turtles reach sexual maturity at approximately 20 years old.
These age estimates coincide with other previous findings
(Nichols, 2001; Marquez 1990).
These
data suggest that the majority of the turtle population in the bay will not be
able to reproduce for several years. Juveniles
in Bahía Magdalena therefore need an extended period of conservation to ensure
their reproductive success; short-term conservation will not be sufficient.
Turtle
Mortality
The
total number of carapaces found in the region is only representative of the
minimum mortality of sea turtles. Since
some turtle mortality was undoubtedly overlooked due to the large sample area
and limited resources, we refer to the dead turtle data gathered as “minimum
mortality” to describe the most conservative mortality estimate (Gardner and
Nichols, 2001). Some places were
only visited once during the year making an exact mortality count impossible.
Over
the course of this study, the dominant species found has shifted from the Black
turtle to the Loggerhead (Fig. 4). This
could be attributed to a decline in Black turtle population, a decrease in the
fishing pressure on Black turtles, or an increase in the consumption of
Loggerheads. The varied species
composition at the different locations suggest that the turtle species harvested
depends upon specific species abundance in popular fishing areas (Fig. 5).
The
areas that consume mostly Loggerheads – Lopez Mateos and Santo
Domingo
– are those that fish outside of Bahía Magdalena where Loggerheads are more
often found. In areas where Black turtle carapaces are most common –
Puerto San Carlos, Puerto Magdalena, Puerto Alcatraz, and Puerto Chalé – the
fishing location tends to be within the bay or estuary, which the Black turtle
uses as a nursery and feeding ground. In
Puerto San Carlos, where most fishermen stay within Bahía Magdalena, 91% of
carapaces recorded are those of Black turtles.
The opposite is true for Santo Domingo, where Loggerheads account for 74%
of the turtle mortality and fishing occurs in the Pacific Ocean.
There
were two forms of mortality involved in this study: consumptive and
non-consumptive. From 2000 to 2002,
the percentage of minimum mortality due to consumption remained relatively
constant. Sea turtle consumption is
a result of cultural traditions in Mexico.
Since consumption also constitutes the large majority of minimum
mortality, it should be a focus for sea turtle conservation and education
efforts. In the case of Puerto San Carlos, where conservation measures
are in progress, there is already a trend of reduced consumption mortality (Fig.
6). From this observation we
recommend increased education and conservation in other areas where sea turtle
consumption is high, such as Puerto Magdalena and Puerto Alcatraz.
The
decrease of the average SCL since 1995 indicates that the turtles caught more
recently are smaller and therefore younger than those caught previously (Fig.
7). The
average SCL data from 2002 may indicate a slight recovery in population size,
but does not break the overall downward trend shown by the regression line (Fig.
8). This
trend could be an indication of over-harvesting of the Black turtle (Royce,
1996).
The
size frequency distribution shows that the majority of dead turtles found were
juvenile. The dominance of juvenile
mortality in the bay indicates that fewer Black turtles are reaching sexual
maturity before being harvested. A
decreasing number of turtles reaching reproductive age will eventually lead to
an overall decrease of turtle populations (Royce, 1996).
Conclusions
Decreasing
average SCL (Fig. 7,8), high consumption rates (Fig. 4,5), and widespread
harvesting of juveniles (Fig. 9) indicate that Black sea turtle populations in
Bahía Magdalena are decreasing. Considering
the current population structure favoring juveniles (Fig. 3) and the relatively
slow growth rates of Black turtles (Fig. 2), immediate and continuing protection
is required to adequately preserve Black sea turtles.
Specifically, we recommend the implementation of conservation measures
such as environmental awareness and education, which have proven effective in
the region (Fig. 6) to slow and
prevent the anthropogenic mortality of sea turtles.
In addition, we recommend the implementation of a marine protected area
in the juvenile nursery ground Estero Banderitas (Fig. 1), to allow time for the
population to recover.
Acknowledgements
We
wish to thank our advisor, Dr. Volker Koch, for his guidance, the students and
staff at the School for Field Studies for their assistance in the field, y
los pangueros de SFS que pasaron buenos ratos con nosotros.
Works
Cited
Alvarado-Diaz,
J. “Evaluation of the Black Sea
Turtle Project in Michoacán, Mexico.” Marine
Turtle Newsletter. Jan 2001.
5-6.
Blanco-Castillo,
Y. “Mexican War to Protect Sea Turtles.” Greenpeace International. 185-188.
SFS Ref. 0862.
Chaloupka,
MY and JA Musick. Age, Growth, and Population dynamics. PL Lutz and JA Musick, ed.
CRC Press, 1997.
Garcia-Martinez,
S and WJ Nichols. “Sea Turtles of
Bahía Magdalena, Baja California Sur, Mexico: Demand and Supply of an
Endangered Species.” Tenth bicentennial Conference of the International Institute of
Fisheries Economics and Trade. Oregon
SU: Corvallis, Oregon, 2000.
Gardner,
SC and WJ Nichols. “Assessment of
Sea Turtle Mortality Rates in The Bahía Magdalena Region, Baja California Sur,
Mexico.” Chelonian Conservation Biology.
4:1, 2001. 197-199.
Gomez,
ED. “Problems of Enforcing Sea
Turtle Conservation Laws in Developing Countries.”
Biology and Conservation of Sea
Turtles. KA Bjorndal, ed.
Smithsonian IP: Washington, 1995. 537-39.
Marquez,
RC. FAO
Species Catalogue, volume 11. Sea
Turtles of the World. Food and
Agriculture Organization of the United Nations. 1990.
Miller,
JD. “Reproduction in Sea
Turtles.” The
Biology of Sea Turtles. Lutz,
PL and JA Musick, ed. CRC Press:
Boston, 1997. 70.
Nichols,
WJ. Doctoral Thesis.
U of Arizona: Tucson, 2001.
DOF,
Diario Oficial de la Federación, México.
Acuerdo por el que se establece
veda para las especies y subspecies de tortuga marina en aguas de jurisdicción
Federal del Golfo de México y Mar Caribe, asi como en las costas del Océano
Pacifico, incluyendo el Golfo de California. May 31, 1990.
Royce,
WF. Introduction
to the Practice of Fishery Science. Academic
Press: San Diego, 1996.
Rubloe,
JA. “Shrimpers and Lawmakers
Collide Over a Move to Save the Sea Turtles.”
Smithsonian. 1989. 45-55.
This page created by: Gray Lyons ('04)
Return to the Gray Lyons Homepage
Return to the Davidson College Homepage