
This web page was produced as an assignment for an undergraduate course at Davidson College.
The Proton-Pump Inhibitor Lansoprazole Enhances Amyloid β Production
Summary:
In this study, Badiola et al. examined the effect of lansoprazole and other proton-pump inhibitors (PPIs), on amyloid-β (Aβ) production both in vitro and in vivo. Aβ production and deposition in the brain has been implicated in the pathology of Alzheimer’s disease (AD). Instead of examining the current drugs for AD or designing new drugs, Badiola et al. examined existing drugs for other conditions for their potential in treating AD. Through a therapeutic performace mapping system (TPMS), Badiola et al. have found that lansoprazole, a drug currently used to inhibit gastric secretions in conditions such as peptic ulcer disease, could be a potential modulator of the process involved in amyloid-β pathology. Therefore, in this study, they were examining the potential of lansoprazole, an existing drug, as a new drug for treating Alzherimer’s disease. The in vitro experiments in this study were performed on cultured Chinese hamster ovary (CHO) cells, that were stably transfected with both wild-type (wt) human amyloid precursor protein (APP) and presenilin 1 (PS1) (PS70 cells). They exposed these cells for 24 hours to a proton pump inhibitor (lansoprazole, omeprazole, pantoprazole, or esomeprazole) at different concentrations. Through this and several subsequent experiments, Badiola et al. presented evidence that lansoprazole increased the production of several Aβ species, while decreasing the production of Aβ38. They concluded that lansoprazole may be achieving its effect by modulating both β-secretase (BACE1) and γ-secretase, proteases acting in Aβ peptide generation. Moreover, in vivo lansoprazole treatments in wild type and AD transfected mice showed resulted in increase amyloid production. The wild type mice were presenting a model for an unaffected organism, while the transfected mice were supposed to model organisms affected by Alzheimer’s disease. Therefore, lansoprazole is not only effective in cell cultures, but is actually able to surpass the blood-brain barrier and show in vivo effects, that may be further studied and used in AD research and treatment.
My Opinion:
Overall, I think that Badiola et al. employed a good overall strategy, going from in vitro studies to in vivo studies, and showing a logical succession of experiments.
However, I think that some of their figures are not very clear. For instance, in Figure 2C, it is not very clear what is significant compared to what. Also, the blots they show in Figure 2 also do not present very clear distinctions between amounts they claim to be different. Perhaps they should have also used a different technique to quantify their results. In addition, they do not explain why they only included the 20 mg/kg treatment in the transgenic mice but not the wild type. Including it into both groups would have present more room for comparison. Moreover, in order to better compare those groups, they also could have mentioned their specific numbers instead of leaving the reader to approximate from the axes. Also, they had a pretty small sample size in their in vivo experiments. Had they used a larger one, they potentially could have observed a significant increase in Aβ42 production instead of just a moderate, non-significant increase.
Figures:
Figure 1:

Figure 1 shows the effect of lansoprazole and other PPIs on Aβ40 and Aβ42 production in vitro. PS70 cells were treated with lansoprazole (250 nM – 50 µM) and similar PPIs (5 uM-50 µM) at different concentrations for 24 hours before measuring Aβ40 and Aβ42 levels in the conditioned media compared to vehicle by ELISA immunoassays. As a negative control, the γ-secretase inhibitor DAPT (N-[N-(3,5-Difluorophenacetyl)-L- alanyl]-S-phenylglycine t-butyl ester, 2µM) was employed. Panel A shows the increase in amyloid production with increasing concentrations of lansoprazole. Aβ40 levels showed an up to 2-fold non-significant increase with concentrations of 10 µM or higher. Aβ42 levels showed a dose-dependent increase between 5 µM and 50 µM, up to a 3-fold increase at 50 µM. Overall, this indicates that lansoprazole treatment increases amyloid production similar to other Aβ inducers, such as caffeine. In Panel B, Badiola et al. examined the effect of other PPIs, omeprazole, pantoprazole, and esomeprazole, at concentrations from 5 µM to 50 µM, to determine if similar PPIs share this feature of Aβ modulation with lansoprazole. The results suggest that these drugs do not have a significant effect on Aβ40 production, but they do have a dose-dependent effect on Aβ42 production, up to a 175 % increase. This suggests that other PPIs also share this feature of Aβ modulation, but their effect may not be homogenous or on the same magnitude as that of lansoprazole.
Figure 2:
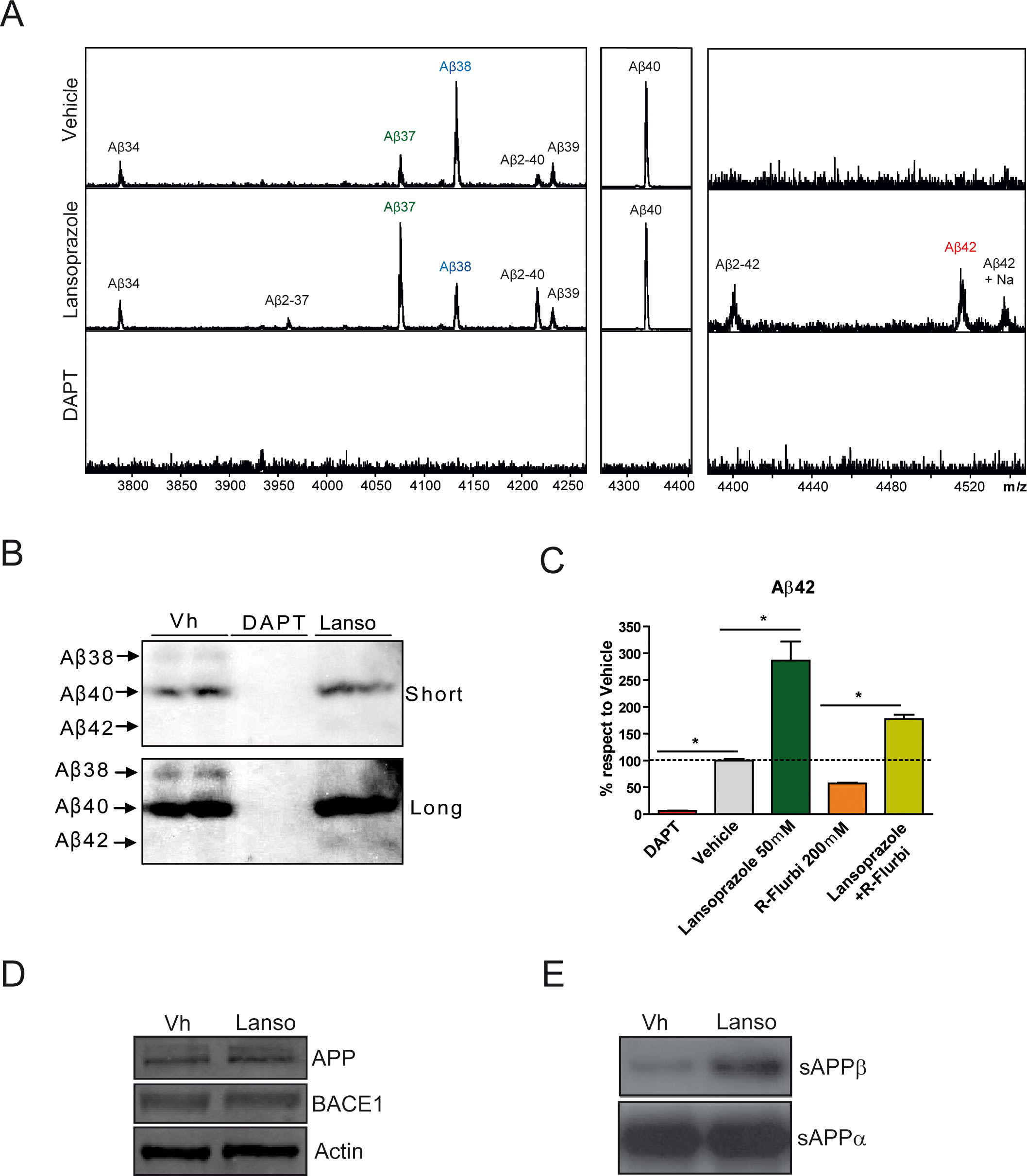
Figure 2 shows the effect of lansoprazole on the production of several Aβ species through different research techniques. Panel A shows the Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) analysis of several Aβ species from conditioned PS70 supernatants after exposure to 50 µM of lansoprazole for 24 hours. Since γ-secretase has multiple APP cleavage sites, different Aβ species are generated, as illustrated in this graph. The graph shows the MALDI-MS for lansoprazole, and also for the vehicle and DAPT. DAPT treatment, as expected, resulted in no generation of Aβ species as γ-secretase was inhibited, thus confirming the use of DAPT as a negative control. The vehicle showed the production of Aβ34, Aβ37, Aβ38, Aβ2-40,Aβ 40, andAβ 39. The lansoprazole treatment was similar, but it also showed the production of Aβ2-37,Aβ 2-42, Aβ42, and Aβ42+Na. It shows increases in Aβ37 and 2-40, while Aβ38 decreased. Moreover, the increase of Aβ2-37, Aβ2-40, and Aβ2-42 species may be indicative of meprin β metalloprotease activity.In panel B, a Western blot analysis of Aβ38, Aβ40 and Aβ42 was performed using the conditioned media obtained from the same preliminart experimental design as in panel A to obtain conditioned media. A decrease in the production Aβ38 and an increase in the production of Aβ42 were observed, as in panel A. From these results, Badiola et al. suggested that lansoprazole may be acting as an inverse γ-secretase modulator (GSM), such that it would achieve its results from shifting the γ-secretase cleavage site to rise Aβ 42 and lower Aβ 38. R-fluriprofen, and NSAID, is a straight GSM, lowering Aβ42, and rising Aβ38, and Badiola et al. examined its interaction with lansoprazole in Figure 2C. ELISA immunoassays were used. Cells were treated either with lansoprazole (50 uM), R-fluriprofen (200 uM), or both, and Aβ42 was measured, and reported as % change from vehicle treatment. As from previous data, lansoprazole treatment resulted in a significant increase in Aβ42 generation, and as previously studied, treatment with R-fluriprofen lowers amyloid production. When combined, lansoprazole was able to counteract the effects of R-fluriprofen and result in a significant increase in Aβ42 production compared to R-FLuriprofen treatment only. Figure 2D shows a Western Blot analysis of APP and BACE1 protein levels, suggesting that lansoprazole has no effect on them. APP can be processed by 2 pathways, the non-amyloidogenic pathway and the amyloidogenic pathway, the latter involving BACE1 activity. Figure 2E shows another Western blot, this time of sAPPβ and sAPPα, and it shows that sAPPβ, a BACE1 cleavage product, increased from treatment with lansoprazole. Therefore, this suggests that lansoprazole does increase BACE1 activity, not by increasing its amount, but pH variations resulting from drug treatment could cause this increase.
Figure 3:
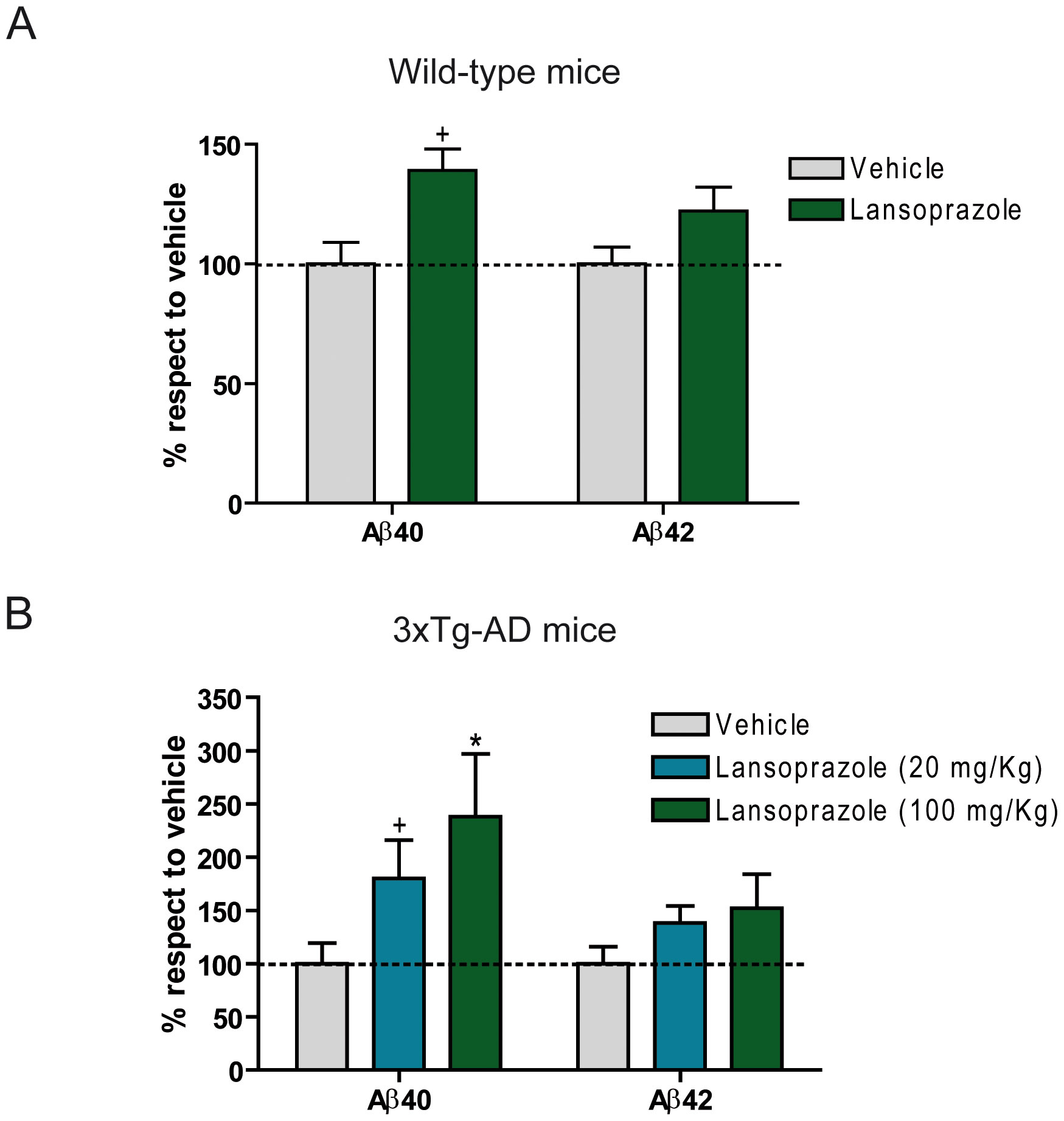
Figure 3 shows the results from the in vivo analysis of the effects of lansoprazole on the production of Aβ40 and Aβ42. The two groups, wild-type (A) and 3xTg-AD transgenic (B) mice were exposed to control (vehicle) or lansoprazole injections for 5 consecutive days. A: Figure 3A shows the wild-type mice Aβ40 production increases significantly from lansoprazole treatment (100 kg/mg), but Aβ42 production does not. B: Figure 3B shows the dose-dependent effect of lansoprazole (20 mg/kg, 100 mg/kg) on Aβ40 production in 3xTg-AD transgenic mice, while there was no significant increase in Aβ42 either. Compared to the wild-type mice, Aβ production was higher, already showing a significant difference at 20 mg/kg, and at 100 mg/kg, they exhibited a 2.5 fold increase compared to an approximately 1.4 fold increase in transgenic mice. The doses examined in this experiment were similar to doses that would be prescribed to humans. For instance, Badiola et al. note that, using body surface area normalization method, the 20 mg/kg and 100 mg/kg doses in mice would convert to 100 mg/day and 486 mg/day, respectively. They mention that the prescribed dose for Zollinger-Ellison syndrome is 180 mg/day and that a recent patent proposes 400 mg/day to inhibit tumor growth, both of these values are between the comparable doses tested in this experiment.
Figure 4:
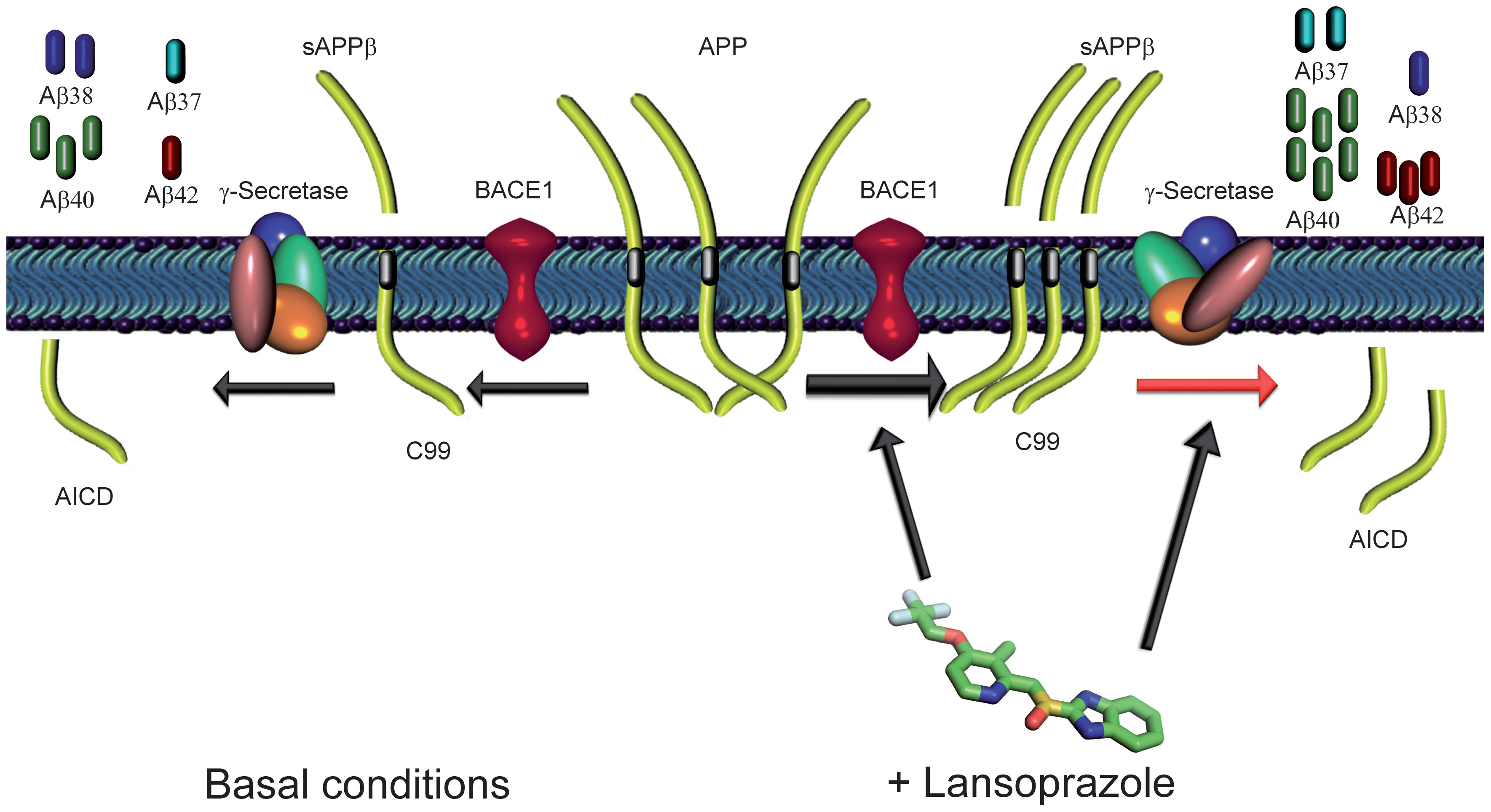
Figure 4 shows a diagram of the proposed mechanism of lansoprazole Aβ production, as suggested by the results of this study. The diagram shows that lansoprazole may function in modulating both BACE1 and γ-secretase, that are employed subsequently in a mechanism to produce Aβ peptides. A first cleavage of APP by BACE1 generates sAPPβ and C99. This step was supported by results in Figure 2E, that showed an increase in vitro of APPβ upon lanzoprazole treatment. In the next step, γ-secretase generates amyloid precursor orotein intracellular domain (AICD) and Aβ peptides. Therefore, increasing BACE1 protease activity results in an overall increase in Aβ peptides. Moreover, Figures 2A and 2B also supported the hypothesis of lansoprazole’s inverse GSM role to modulate γ-secretase to increaseAβ 42 production, while decreasing Aβ 38 production.
References:
Frizzi Bschorer's Genomics Page
Genomics Page
Biology Home Page
Email Questions or Comments.
© Copyright 2013 Department of Biology, Davidson College, Davidson, NC 28035