

Interferon gamma (IFN-g) is a dimeric glycoprotein involved in both the adaptive and innate immune responses. This 140 amino acid homodimer was named because of its ability to block or interfere with viral replication or lead to the elimination of virus from infected cells without actually killing them. IFN-is a member of the cytokine family of proteins, and its main functions include macrophage activation, upregulation of certain T helper cells, and enhanced antigen presentation by MHC molecules.
![]() What
is the structure of IFN-g?
What
is the structure of IFN-g?
According to a study of the crystal structure of the IFN-g:receptor complex, "each domain of the IFN-g homodimer consists of six helices related by a non-crystallographic twofold axis" (Walter et al., 1995).
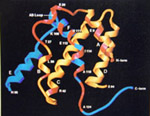
Click here for legend and enlarged
image
![]() What type of cells produce IFN-g ?
What type of cells produce IFN-g ?
IFN-g is secreted by activated natural killer (NK) cells, cytotoxic T cells, the first subset of T helper cells (TH1), and TH0 cells. Although the majority of IFN-g is produced by the helper T cells, for each cell type, the level of secreted protein is thought to be regulated by a negative feedback mechanism. This has been studied in detail in CD4+ (TH1 cells), where researchers have been able to "define a mechanism of cellular desensitization where a cytokine down-regulates expression of a receptor subunit required primarily for signaling and not ligand binding" (Bach et al., 1995) by studying the interactions between IFN-g and the beta subunits ofs its recepor molecules.
![]() What
are the receptor molecules for IFN-g and how is the protein:receptor complex
formed?
What
are the receptor molecules for IFN-g and how is the protein:receptor complex
formed?
The surface proteins CD119 and IFNGR2 are the primary receptor molecules for the IFN-g ligand. The former is expressed by macrophages, monocytes, B cells, and endothelial cells. To form the protein:receptor complex, a minimum of two subunits of IFNGR2 must be present. Associated with the intracellular domains of each are the Janus protein kinases Jak 1 and Jak 2, which "are brought together and activated by phosphorylation by the binding of INF-g to the receptor complex" (Newport et al., 1996). It is believed that both subunits are required for normal signal transduction.
![]() What
cell types can INF-g interact with? What effect does IFN-g have on
these lineages?
What
cell types can INF-g interact with? What effect does IFN-g have on
these lineages?
The effects of IFN-g are seen in B cells, T cells, macrophages, natural killer cells, endothelial cells, dendritic cells, and some somatic cells. (Janeway et al., 1999)
B cells: IFN-g levels help regulate B cell differentiation and IgG2a synthesis, mainly by facilitating isotype switching (Clark, 1999).
T cells: IFN-g has been shown to inhibit the growth of TH2 cells and promote the growth of TH1 cells.
Macrophages: IFN-g secretion leads to the activation of macrophages, an increase in their recognition of foreign peptide by antigen presenting cells (APCs) ie. MHCI and MHCII and enhanced phagocytotic activity.
Natural killer cells: IFN-g is not only secreted by NK cells, but is capable of inducing NK cell activation, which is an important part of antibody dependent cell mediated toxicity (ADCC) and the development of innate immunity to viruses and other intracellular pathogens.
Endothelial cells:IFN-g "can act synergistically withTNF-alpha when released from an activated T cell to change the shape of endothelial cells, allowing increased blood flow, vascular permeability, and emigration of leukocytes, fluid, and protein into [an] infection site" (Janeway et al., 1999).
Dendritic cells: According to a study in Hepatitis Weekly, IFN-g can upregulate the APC capability of MHCII molecules in splenic dendritic cells in transgenic mice. (Henderson, 1996)
Somatic cells: IFN-g can help increase the antiviral capabilities of somatic cells by increasing the MHCI and MHCII functions, thus increasing presentation of viral peptides by APCs.
![]() What
happens when an organism has a mutant form of or is missing the gene that
codes for IFN-g?
What
happens when an organism has a mutant form of or is missing the gene that
codes for IFN-g?
Gene knockout studies conducted in mice have shown that organisms that lack interferon-gamma are highly suseptible to intracellular pathogens such as M. tuberculosis. (Janeway et al., 1999) "Mice in which the gene for interferon-gamma has been disrupted have defective production of macrophage antimicrobial products and reduced expression of major-histocompatibility -complex class II antigens and die of disseminated mycobacterial infection" (Newport et al., 1996). Thus, the lack of this cytokine puts IFN-g non-producers at an increased risk to develop a mycobacterial infections. Gene knockout studies such as this have been extremely helpful in broadening our knowledge of the role cytokines play in immunity and the development of the potential treatment options IFN-g has to offer.
![]() Does
IFN-g have any treatment applications?
Does
IFN-g have any treatment applications?
There is currently a lot of research being done into the medicinal capabilities of IFN-g in diseased patients. Here are just a few of the diseases that IFN-g may be used to help control or treat in the future:
![]() Hepatitis
B Virus (HBV): "Immunohistochemical staining has shown that treatment
of transgenic mice with mouse recombinant IFN-g daily for 6 consecutive
days resulted in an increased expression of Ia antigen on splenic dendritic
cells" (Akbar et al., 1996).
Hepatitis
B Virus (HBV): "Immunohistochemical staining has shown that treatment
of transgenic mice with mouse recombinant IFN-g daily for 6 consecutive
days resulted in an increased expression of Ia antigen on splenic dendritic
cells" (Akbar et al., 1996).
![]() Tuberculosis
(TB): Due to its primary action of macrophage activation, INF-g
was shown to be effective in treatment of patients with mycobacterial infections
such as TB. Researchers believe the "mechanisms of action of INF-g
in the treatment of mycobacterial infections may include alteration of
intracellular compartments, enhanced display of surface molecules, or increased
antibiotic concentrations" (Key, 1994).
Tuberculosis
(TB): Due to its primary action of macrophage activation, INF-g
was shown to be effective in treatment of patients with mycobacterial infections
such as TB. Researchers believe the "mechanisms of action of INF-g
in the treatment of mycobacterial infections may include alteration of
intracellular compartments, enhanced display of surface molecules, or increased
antibiotic concentrations" (Key, 1994).
![]() Osteopetrosis: According to the New England Journal of Medicine,
recombinant
human IFN-g has potential as a long-term treatment for osteopetrosis,
for it was shown to "increase bone resorption and hematopoiesis and improve
leukocyte function" (Key et al., 1995). Interestingly, the authors
of this study hypothesized that IFN-g was successful in the treatment of
osteopetrosis because it stimulated the production of 'osteoclastic superoxide',
which would then increase the degradation of bone matrix by osteoclasts,
the subset of cells whose function is often impaired in patients with this
disease.
Osteopetrosis: According to the New England Journal of Medicine,
recombinant
human IFN-g has potential as a long-term treatment for osteopetrosis,
for it was shown to "increase bone resorption and hematopoiesis and improve
leukocyte function" (Key et al., 1995). Interestingly, the authors
of this study hypothesized that IFN-g was successful in the treatment of
osteopetrosis because it stimulated the production of 'osteoclastic superoxide',
which would then increase the degradation of bone matrix by osteoclasts,
the subset of cells whose function is often impaired in patients with this
disease.
![]() Malaria: According to an abstract submitted to the 7th Malaria
Meeting of the British Society for Parasitology held in September of 1995,
"IFN-g delivered [via salmonella vector] promoted control of the blood-stage
infection of P. chabaudi in mice . . .when innoculated orally either
0,3, or 8 days before the malaria infection" (Marble, 1996).
Malaria: According to an abstract submitted to the 7th Malaria
Meeting of the British Society for Parasitology held in September of 1995,
"IFN-g delivered [via salmonella vector] promoted control of the blood-stage
infection of P. chabaudi in mice . . .when innoculated orally either
0,3, or 8 days before the malaria infection" (Marble, 1996).
![]() Atopic Dermatitis: "Evaluation of early atopic dermatitis legions
had shown a predominance of TH2
cells with a relative deficiency of TH1
cells" (Guttman, 1997). Since IFN-g is known to inhibit TH2
cells and promote TH1 cell growth,
researchers investigated the use of the glycoprotein as a means by which
to "selectively modify the existing immune response and restore it to its
normal balance" (Guttmann, 1997). IFN-g was shown to be more
effective than the current treatment of steroid administration. As
of 1998, IFN-g treatment of atopic dermatitis was in Phase III trials.
Atopic Dermatitis: "Evaluation of early atopic dermatitis legions
had shown a predominance of TH2
cells with a relative deficiency of TH1
cells" (Guttman, 1997). Since IFN-g is known to inhibit TH2
cells and promote TH1 cell growth,
researchers investigated the use of the glycoprotein as a means by which
to "selectively modify the existing immune response and restore it to its
normal balance" (Guttmann, 1997). IFN-g was shown to be more
effective than the current treatment of steroid administration. As
of 1998, IFN-g treatment of atopic dermatitis was in Phase III trials.
![]() Cancers:
Research done by Wakimoto et al. and Abdelwahab et al. indicates
that IFN-g may eventually be used in gene therapy or in the development
of a cancer vaccine, respectively. According to a study published
in Cancer Biotechnology Weekly, "transduction with interferon-gamma gene
resulted in a prominent increase in theraputic efficacy of CTL [cytotoxic
T lymphocytes] in both metastatic and subcutaneous tumor models" (Wakimoto
et al., 1996). These findings may provide a possible strategy for
adoptive immunotherapy for human cancers. Similarly, a study
published in Cellular Immunology reported that "tumor cells transduced.
. . with IFN-g genes stimulated a potent and specific antitumor immunity
in experimental animals" (Abdelwahab et al., 1996). Therefore, according
to these results, it may be possible to develop a vaccine using IFN-g gene-modified
melanoma cells with the potential to increase the the body's immune response
to cancers when used in conjunction irradiation treatments.
Cancers:
Research done by Wakimoto et al. and Abdelwahab et al. indicates
that IFN-g may eventually be used in gene therapy or in the development
of a cancer vaccine, respectively. According to a study published
in Cancer Biotechnology Weekly, "transduction with interferon-gamma gene
resulted in a prominent increase in theraputic efficacy of CTL [cytotoxic
T lymphocytes] in both metastatic and subcutaneous tumor models" (Wakimoto
et al., 1996). These findings may provide a possible strategy for
adoptive immunotherapy for human cancers. Similarly, a study
published in Cellular Immunology reported that "tumor cells transduced.
. . with IFN-g genes stimulated a potent and specific antitumor immunity
in experimental animals" (Abdelwahab et al., 1996). Therefore, according
to these results, it may be possible to develop a vaccine using IFN-g gene-modified
melanoma cells with the potential to increase the the body's immune response
to cancers when used in conjunction irradiation treatments.
As was described above, much of the
research being done into the use of the IFN-g gene for treatment shows
promise for the future of gene therapy. More recently, studies involving
the experimental visceral leishmaniasis have found that "a transferred
cytokine gene (IFN-g) can reach sites of infection, be properly expressed,
and induce intracellular antimicrobial activity in the tissues" (Taylor
et al., 1998). In addition, some researchers believe that an assay utilizing
IFN-g may be superior to the tuberculin skin test for the detection and
diagnosis of the mycobacterium tuberculosis infection. (Boyles et al.,
1997) This further supports the treatment potential and the impact
IFN-g may have in the realm of the medical community.
For these reasons and much, much more, interferon-gamma is my favorite
protein involved in the immune response.
REFERENCES:
Abdelwahab, Z.; Dar, M.M.; Hester, D.; Vervaert, C.; Gangavalli, R.; Barber, J.; Darrow, T.L.; Seigler, H.F. (1996) Effect of Irradiation on Cytokine Prodution, MHC Antigen Expression, and Vaccine Potential of Interleukin-2 and Interferon-gamma Gene-Modified Melanoma Cells. Cellular Immunology 171(2):246-254.
Akbar, S.M.F.; Inaba, K.; Onji, M. (1996) Upregulation of MHC Class II Antigen on Dendritic Cells From Hepatitis B Virus Transgenic Mice by IFN-g: Abrogation of Immune Response Defect to a T-cell Dependent Antigen. Immunology 87(4): 519-527.
Bach, E.A.; Szabo, S.J; Dighe, A.S.; Ashkenazi, A.; Aguet, M.; Murphy, K.M.; Schreiber, R.D. (1995) Ligand-Induced Autoregulation of IFN-g Receptor Beta Chain Expression in T Helper Cell Subsets. Science 270(2):1215-1217.
Boyles, S.; Key, S.W. (1997) IFN-g has advantages over TST in HIV+ patients. AIDS Weekly Plus (8/11897):15.
Clark, C.; Boyles, S. (1999) Recombinant Interferon provides B cell help. Blood Weekly (3/8/99):3.
Guttman, C.; (1997) Interferon-gamma may be next treatment option for Atopic Dermatitis. Dermatology Times 18(2):13.
Janeway, C.A.; Travers, P.; Walport, M.; Capra, J.D. (1999)
ImmunoBiology: The Immune System in Health and Disease.
4th edition. Elsevier Science Ltd/Garland Publishing. New
York, NY. 288, 385-6, 397, 438.
Key, K. (1994) NIH reports on treatment of TB with IFN-gamma. AIDS Weekly (11/14/98): 19.
Key, L.; Rodriguiz, R.; Willi, S.; Wright, H.; Hatcher, H.; Eyre, D.; Cure, J.; Griffin, P.P.; Ries, W.L. (1995) Long-Term Treatment of Osteopetrosis with Recombinant Human Interferon Gamma. New England Journal of Medicine 332(24): 1594-9.
Marble, M. (1996) Malaria vaccines. Malaria Weekly(11/18/96): 8.
Newport, M.J.; Huxley, C.M.; Huston, S.; Hawrylowicz, C.; Oostra, B.; Williamson, R.; Levin, M. (1996) A Mutation in the IFN-g Receptor Gene and Suscepibility to Mycobacterial Infection. New England Journal of Medicine 335(26): 1941-8.
Taylor, A.P.; Murray, H.W. (1998) Theraputic Effect of Interferon-gamma Gene Transfer in Experimental Visceral Leishmaniasis. Journal of Infectious Diseases 178(3):908-11.
Wakimoto, A.J.; Tsunoda, R.; Okabe, S.; Yoshida, Y.; Aoyagi, M.; Hirakawa, K.; Hamada, H. (1996) In Vivo Antitumor Effect of Cytotoxic T Lymphocytes Engineered to Produce Interferon-gamma by Adenovirus-Mediated Genetic Transduction. Biochemical and Biophysical Research Communications 218(1):164-170.
Walter, M.R.; Windsor, W.T.; Nagabhushan, T.L.; Lundell, D.J.; Lunn,
C.A.; Zauodny, P.J.; Narula, S.K. (1995) Crystal structure
of a complex between interferon-gamma and its soluble high-affinity receptor.
Nature 376(7):230-235.

© Copyright 2000 420 Beaty St. Davdison, NC