This web page was produced as an assignment for an undergraduate course at Davidson
College.
Type 1 Diabetes: An
Autoimmune Disease
Page
Contents
Brief
Introduction: What is Insulin-Dependent Diabetes Mellitus?
Immunological
basis of Diabetes
Humoral
Autoimmunity
Cell
Mediated Autoimmunity
Genetic
and Environmental Causes of Diabetes
Possible
treatments for Diabetes
References
What
is Insulin-Dependent Diabetes Mellitus?
Type I diabetes
mellitus - otherwise known as juvenile diabetes or insulin-dependent diabetes
mellitus (IDDM)- is considered to be an autoimmune disease. This disease
usually begins in childhood or in the young adult years and tends to be more
prevalent among females than males (InteliHealth 1999). Accounting for
only 5% or less of diabetes in the United States, IDDM is not the most common
form of diabetes. However, the physiological effects of IDDM tend to have
a much greater impact upon patients' lives than the more common adult-onset form
of diabetes known as non-insulin-dependent diabetes (NIDDM) (InteliHealth
1999). Thus, a thorough understanding IDDM and the possible methods
of prevention and treatment of this disease is of utmost importance.
Simply put, IDDM results when the immune system attacks and destroys the
insulin-producing ß cells of the
pancreas. The results of this attack are a pancreas that produces little or
no insulin (Fig 1) and an inability to regulate the level of sugar in the blood
(Scriver et al., 1995). Research shows that a great
majority of people with IDDM inherit traits that put them at risk for this
disease. However, studies also show that not everyone who inherits these traits develops
type 1 diabetes. Therefore, it is hypothesized that environmental factors trigger the immune system to destroy the insulin-producing cells.
In a few cases, researchers have been able to link the onset of diabetes with a
viral infection (InteliHealth 1999). However, in most cases, the trigger for
diabetes and the exact cause and mechanism
of this autoimmune attack on the ß cells is not completely understood.
The immunological basis of these topics will be the primary focus of this
website.
|
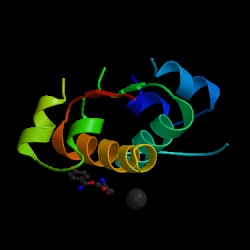
|
Fig 1. Structure of
insulin. Type 1 diabetes is characterized by the absence of
insulin from the body. This lack of insulin presents a problem
because insulin is needed by the body in order to allow sugar to pass
into the cells for energy (Vallence-Owen 1975). Figure courtesy of the
Protein Data Bank. See references
for a link to the page where this image can be viewed at the Protein
Data Bank. |
Introduction
to Immunology of Insulin-Dependent Diabetes Mellitus (IDDM)
Use of animal models:
Several animal models of
the human autoimmune disease IDDM have helped us understand the mechanism of
this disease and need to be explained at this time. The NOD (non-obese
diabetic) strain of mice spontaneously develops IDDM and is a useful tool for
studying this disease. Diabetes in these mice involves a progressive
mononuclear cell infiltration in the pancreatic islets of the mice. The
infiltration begins at about four weeks of age and leads to ß cell destruction
and hyperglycemia (Trembleau et al., 1999). The pattern of disease in these mice
is very similar to that in humans except for the degree of incidence between the
sexes. In the NOD mouse, 70 - 80% of females develop diabetes while only
10 - 20% of males develop this disease (Trembleau et al., 1999). The BB (BioBreeding) rat also
spontaneously develops diabetes and the pattern of this disease is very similar
to IDDM in humans with the exception of the association of T cell lymphopenia
with the BB rat (Delves et al., 1998).
The
general finding that endocrine tissue is especially vulnerable to autoimmune mechanisms
led researchers to hypothesize that autoimmunity plays a pathogenic role in the
development of type 1 diabetes (IDDM) (Andersen 1980). Indeed,
research has demonstrated that IDDM is an
autoimmune disease in which specific T cells selectively destroy
the insulin-producing ß cells of the pancreatic islets (Janeway et al.,
1999). Several lines of evidence support this important determination. First,
it has been shown in human patients with IDDM that lymphocytic infiltrates (known
as insulitis)
containing activated T lymphocytes are located around pancreatic islets in patients who die shortly after being
diagnosed with type 1 diabetes. In addition to studies in humans, animal
models have also confirmed the finding of insulitis surrounding islets of
diabetic animals. Additionally, in the BB rat and the NOD mouse, islet
cell surface autoantibodies (ICSA) have been identified. Research has also
indicated that immunosuppressive drugs are readily able to delay or prevent the development
of disease in BB rats or NOD mice (Andersen 1980). Lastly, a strong association exists
between type 1 diabetes and other autoimmune diseases such as vitiligo and
pernicious anemia, and many patients with type 1 diabetes have
family histories characterized by the prevalence of autoimmune disease (Scriver
et al, 1995). The mechanism by which the ß cells are
selectively destroyed is of utmost interest and has been highly researched;
however the immunological basis of this disease is not yet fully understood. IDDM has been
described to proceed in four distinct stages: (1) pre-clinical ß cell
autoimmunity, (2) onset of clinical diabetes, (3) transient remission, and (4)
established diabetes associated with acute and chronic complications and
premature death (Rewers et al., 1997). The first of these steps will be
the main focus of this website because it is during this stage of diabetes that
the pancreatic ß cells are selectively destroyed by the immune system.
back
to top of page
Humoral
Autoimmunity of Insulin-Dependent Diabetes Mellitus (IDDM)
Researchers have determined that
during the first stage of IDDM, antibodies are synthesized that act against the insulin-producing cells of the
pancreas (Scriver et al., 1995).
The consequence of these autoantibodies is a destruction of the insulin-producing beta cells of the islets of Langerhans’ cells and an absence or deficiency of circulating
insulin (Slavkin 1999). This destruction probably occurs in genetically
susceptible individuals in response to a particular environmental agent.
It appears that ß cell-autoimmunity develops in less than 5% of the population
and progresses into full diabetes in less than 1% of the general population
(Slavkin 1999). The following specific autoantibodies have been discovered:
Islet Cell Cytoplasmic Antibodies:
Indirect immunofluorescence stains of human pancreas sections demonstrate that nearly 90%
of recently diagnosed diabetics have islet cell cytoplasmic antibodies (ICCA).
ICCA are present in only 0.5 - 4% of non-diabetic individuals (Scriver
et al., 1995). Although research indicates that ICCA are not specific for ß cells
(and thus recognize antigens present in other cell types of the islet), the
autoimmune attack of these antibodies appears to destroy ß cells
selectively. It is interesting to note that the concentration of these autoantibodies decreases with
time; research indicates that approximately two years after an initial
diagnosis of IDDM, about 90% of patients no longer have detectable levels of
anti-islet antibodies. The few individuals with persistent anti-islet
antibodies may represent a subpopulation of persons with a more generalized
autoimmune defect (Scriver et al., 1995).
Islet Cell surface Antibodies: Eighty
percent of diabetic individuals also have autoantibodies directed against islet
cell surface antigens (ICSA) at the time of diagnosis (Scriver et al.,
1995). These ICSAs can lead to lysis of the islet cells in the presence of
complement and tend to be IgM and IgG antibodies (Andersen 1980). As with
ICCA discussed previously, ICSA also decrease considerably after the initial
diagnosis. A recent interesting finding is that ICSA has also been found
in some patients with non-insulin dependent diabetes mellitus (type 2 diabetes
or NIDDM). Thus, it is probable that autoimmune mechanisms can cause
either IDDM or NIDDM. IDDM involves total or almost total destruction of ß
cells, while NIDDM results in partial destruction of the ß cells.
* * * * * * * * * * * * * * * *
Currently, research
is aimed at identifying the multiple antigens within the islet cells with which
these anti-islet antibodies react. As reflected in the categories below, some
progress has been made toward identifying these antigenic targets.
Antibodies to glutamic acid and decarboxylase:
The enzyme glutamic acid decarboxylase (GAD) has been identified within the
human islet cells. Further research determined that 80% of patients with
newly diagnosed IDDM have anti-GAD antibodies while only 2% of normal
individuals have these anti-GAD antibodies. The presence of anti-GAD
antibodies has become a predictor of future development of IDDM in high risk
populations (Scriver et al., 1995).
Autoantibodies to insulin receptors: Anti-insulin
receptor autoantibodies have also been identified in individuals diagnosed with
IDDM and in their relatives who are at risk for IDDM (Scriver et al.,
1995). These antibodies are predominantly IgG with some IgM activity as
well. In a study investigating insulin receptors on diabetic patients'
circulating monocytes, researchers observed severe insulin resistance due to
antibodies to insulin receptors. It was also found that when normal
insulin receptors were exposed to serum from the diabetic patients in the
previous study the insulin-binding defect was reproduced (Andersen 1980).
Antibodies to bovine serum: Interestingly,
many patients recently diagnosed with IDDM have elevated levels of antibodies to
bovine serum albumin. Most patients also have an increased number of
antibodies directed toward a 17-amino-acid epitope in the albumin
molecule. Antibodies to this peptide cross react with a protein located on
the surface of pancreatic ß cells. These findings led researchers to the
interesting hypothesis that ingestion of cow's milk leads to an immune response
to bovine serum albumin in susceptible persons. The epitope in the bovine
serum albumin molecule appears to mimic the structure of the surface protein of
the pancreatic ß cells, and thus the anti-bovine serum albumin antibody is also
an islet cell surface autoantibody. Therefore, a rather controversial
hypothesis is that through a process known as molecular mimicry, intake of cow's
milk, which contains bovine serum albumin, is an environmental element that
precipitates the development of IDDM in genetically susceptible persons (Scriver
et al., 1995).
The autoantibodies
described above provide excellent markers for the destruction of pancreatic ß cells
and are useful in predicting the future development of type 1 diabetes
mellitus. It is possible that these autoantibodies may have a primary causal
role in the ß cell destruction observed in IDDM. However, it is also
equally likely that as a result of ß cell destruction, ß cell antigens are
released into circulation, thereby inducing anti-islet antibodies. Most
evidence supports the latter of these two hypotheses and suggests a primary role
for cell-mediated autoimmunity (Scriver et al., 1995). As discussed
below, it is probable that pancreatic ß cells are specifically targeted and destroyed by CD8 T cells
(killer T cells) (Janeway et al., 1999).
back
to top of page
Mechanism
of Cell-Mediated Autoimmunity in IDDM
Distinct cell types including alpha, beta, and delta cells are found
within the islets of Langerhans in the pancreas. Each of these cell types
secretes distinct hormones and expresses various tissue-specific proteins (Janeway
et al., 1999). Alpha cells secrete glucagon, beta cells secrete insulin,
and the delta cells are responsible for secretion of somatostatin.
Research suggests that during the disease course of type 1 diabetes, self
antigens unique to the ß cells are presented via MHC class I molecules. These
autoantigens are then recognized by effector CD8 T cells and selectively
destroyed. Thus because only the ß cells are eliminated by the CD8 T
cells, alpha and delta cells still produce glucagon and somatostatin,
respectively. However, the body's ability to make insulin is severely
impaired (Janeway et al., 1999). Research has also determined that CD4 T
cells (also known as helper T cells) may play a role in IDDM. This
suggestion would be consistent with the previous findings that particular MHC
class II alleles render some individuals more susceptible to the disease (see
the section: Genetic
Causes of ß cell Autoimmunity and Diabetes). Again, identifying the autoantigens recognized by CD8 T cells is of
utmost importance. Identifying these antigens will allow scientists to gain a
greater understanding of the disease, thereby increasing their ability to
discover effective treatments or preventative measures for IDDM (Janeway et
al., 1999).
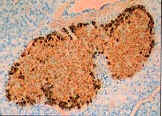
Figure 2 shows the islets of Langerhans from non-diabetic (left)
and diabetic (right) individuals. These pancreatic cells have been stained
for insulin (brown) and glucagon (black). As can be seen in the photos, in
the normal islets of Langerhans, the ß cells are able to produce insulin and
the cells are highly stained with brown. However, in the diabetic
individual on the right, it can be seen that little insulin is produced due to
the selective loss of the ß cells, and thus significantly decreased amounts of
brown staining can be detected. It should also be noted that the islets of
the diabetic patient on the right are stained in black, thereby confirming the
notion that the alpha cells are spared while the ß cells are selectively
destroyed (Janeway et al., 1999).
|
|
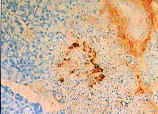
|
|
Fig.
2. Islets of Langerhans cells from a
normal individual. Brown staining confirms presence of insulin from ß cells.
Black staining indicates the presence of glucagon made by alpha cells
(Janeway et al., 1998). |
Islets of Langerhans cells from a
type 1 diabetic. Black staining indicates the presence of glucagon
made by alpha cells. Extreme decrease in the level of brown staining
demonstrates lowered levels of insulin due selective destruction of ß
cells
(Janeway et al., 1998).
|
|
Photos
courtesy of Janeway C, Travers P, Walport M, Capra JD. Immunobiology: the Immune System in Health and Disease. 4th ed. London:Current
Biology Publication;1999. p 508. |
Before approximately
1995 it was thought that IDDM was a direct
result of a defect in CD8 T cells (killer T cells). However, research
under the leadership of Denise Faustman of Harvard University demonstrated that
the defect could be found not within the CD8 cells, but within the MHC class I
molecules (Faustman et al., 1995). The defect in these MHC class I molecules causes the T cells to
act inappropriately and respond to self antigens presented on the ß cells of
the islets of Langerhans (Faustman et al., 1995). As discussed in the
section below, it has been determined that genetic alterations on chromosome six
in MHC class II molecule genes have been identified in a significant number of
diabetic individuals. This information initially appeared contradictory to
Faustman's finding that MHC class I molecules were instrumental in causing an
autoimmune response. However, the researchers then discovered that the
genes for the Tap-1 and Tap-2 proteins were located within the region encoding
the MHC class II molecule. The Tap-1 and Tap-2 proteins are critical in
the assembly of the MHC class I molecule. Thus, Faustman concluded that
the deficiency in the MHC molecule leading to a CD8 T cell autoimmune attack
could be due to a defect in the Tap-1 and Tap-2 genes (Faustman et al,
1998). However, subsequent research has not been able to identify any
point mutations or deletions in the Tap genes. Faustman and her co-workers
argue that the genetic defect in these Tap genes may be more subtle and
complicated than a mutation or deletion. She suggests that simple
variations in these genes may make them incompatible with other genes in the
region. This suggestion implies that variations in MHC class I molecules
will have unpredictable effects, and this idea is exactly what is observed in
the realm of autoimmune diseases. For example, often a variety of
autoimmune diseases are prevalent in a family. Variations in MHC class I
alleles may explain why several autoimmune diseases such as rheumatoid
arthritis, multiple sclerosis, or diabetes can be found within a single family (Faustman
et al., 1995).
Additionally,
it is becoming widely apparent that cytokines play an integral role in the
development of type 1 diabetes. Research indicates that both IFN-alpha and
IFN-gamma are closely associated with IDDM in both human and in NOD mice
(Baldeon et al., 1998). Recent studies have demonstrated that
IFN-gamma acts directly to decrease insulin production and upregulate cell
surface expression of MHC class I molecules in pancreatic ß cells, thereby
amplifying the insulitic process. However, studies involving IFN-alpha do
not suggest similar activity for this cytokine. The action of IFN-alpha is
hypothesized to occur during the stages before the onset of diabetes. It
is proposed that the expression of IFN-alpha in response to potential
diabetogenic stimuli (such as viruses as discussed below) may trigger insulitis
to begin. Because IFN-alpha is also known to be associated with the
stimulation of natural killer cells and T helper 1 cells, early IFN-alpha
expression by ß cells may play a critical role in the development of IDDM
(Baldeon et al., 1998). Interestingly other cytokines such as IL-10
and IL-12 are also intimately associated with the development of IDDM (Balasa et
al., 1998). Research in this area has shown that IL-10
is essential for the early phase of diabetes in NOD mice, but later protects
against the development of this disease. The mechanism of its action is
not yet clear, however, scientists have shown that IL-10 is able to affect the
disease process of NOD mice via the CD8+ T cell pathway without B cells present
as antigen presenting cells and without the need for the CD40-CD40 ligand
pathway. These studies suggest that it is possible that during the early
stages of diabetes, IL-10 may act as a chemoattractant and a differentiation
factor for CD8+ T cells that are specific for ß cell peptides.
However, during the late stages of diabetes, IL-10 may inhibit the generation of
pathogenic CD4+ T helper 1 cells (Balasa et al., 1998).
Additionally, the lack of IL-12 also appears to play a role in the onset of
diabetes (Trembleau et al., 1999). Studies indicate that IL-12
deficient NOD mice are unable to develop a regulatory pathway that is able to
counteract diabetogenic T helper 1 cells and readily develop IDDM (Trembleau et
al., 1999). Research in the area of cytokines is currently very
intense and may provide invaluable information regarding the mechanism of type 1
diabetes development.
back
to top of page
Genetic causes of ß cell
Autoimmunity and Diabetes
As mentioned previously, the development of IDDM is controlled by
several genetic loci, of which the most significant contributor may be the major
histocompatibility complex (MHC) located on chromosome six. Specifically, it has been found
that the primary locus of susceptibility to IDDM includes the, HLA-DR and HLA-DQ
genes, but new possible loci for IDDM outside of the HLA region are currently
being identified (Rewers et al., 1997). Studies involving the BB rat
demonstrate that susceptibility to diabetes is strongly linked to genetic
markers for the MHC and the inheritance of phenotypic markers like T lmyphopenia
(Delves et al., 1998). It is not yet known which of these genetic
markers associated with diabetes are important for development of ß cell
autoimmunity and which determine progress to full scale diabetes (Rewers et
al.,
1997). For example, three out of the fifteen loci linked to type 1
diabetes in NOD mice lead to autoimmunity without the progression of
diabetes. Similar experiments have not yet been performed in humans (Rewers
et al., 1997). Researchers have been unable to identify a particular HLA
genotype that is associated with the initiation of ß cell autoimmunity in
humans. However, it has been determined that the HLA-DRß1*0301/04,
HLA-DQß1*0201/0302 genotype promotes autoimmunity persistence and progression to
the disease state of diabetes. Although the DRß1*0301/04, DQß1*0201/0302
heterozygotes make up only 2% of the population, this genotype is found in
30-40% of IDDM patients. The genetic nature of diabetes continues
to perplex scientists as both susceptibility and protection from IDDM are
associated with changes in the sequence of amino acids even within one locus (Janeway
et al., 1999). For
example, the human HLA-DQß1 gene, the genes *0302
and *0201 are found to be linked to progression of autoimmunity, while other
genes such as *0602 inhibit progression from autoimmunity to diabetes (Rewers et
al., 1997).
In most non-diabetic persons, position 57 of the
HLA-DQß1 chain contains an aspartic acid residue (Fig. 3) (Janeway et al.,
1999). However,
Caucasians patients with IDDM show an increased likelihood of having valine,
serine, or alanine at this position. In non-diabetic
individuals with an aspartic acid residue at position 57, a salt bridge is able
to form to an arginine residue in the adjacent alpha chain of the MHC class II
molecule (Fig. 4). In patients with IDDM, a substituted amino acid at
position 57 to an uncharged residue such as alanine disrupts the stability of
the MHC class II molecule and interferes with the salt bridge formation (Fig. 5)
(Janeway et al., 1998). Additionally, as discussed in the previous
section, some research has found that slight variations in the genes for the Tap
transporter protein, which are located within the region of the MHC class II
molecule may play an integral role in causing an autoimmune reaction by CD8 T
cells to self peptides on ß cells (Faustman et al., 1995).
Obviously, further research in this area needs to be conducted in order to determine the
genotype of many other individuals with ß cell autoimmunity. This
knowledge may then allow scientists to determine the role of HLA and additional
IDDM candidate genes in the induction of autoimmunity and progression to
diabetes (Rewers et al., 1997).
|
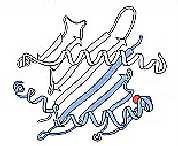
|
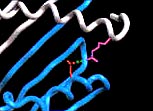
|

|
|
Fig.
3. Position 57 (shown in red) of the
HLA-DQß chain (blue) plays an integral role in susceptibility to IDDM
(Janeway et al., 1998). |
Fig.
4. An MHC class II molecule (from a
non-diabetic individual) with an aspartic acid residue at position 57 is
able to form a stable salt bridge (green) between an aspartic acid residue
(red) and an arginine residue (pink) of the adjacent alpha chain (gray).
An aspartic acid residue at this position is associated with resistance to
IDDM
(Janeway et al., 1998). |
Fig.
5. An MHC class II molecule from a
type 1 diabetic has a substituted amino acid at position 57. This
photo shows alanine (yellow) at position 57. The presence of alanine
at this position disrupts the formation of the salt bridge and is
associated with increased susceptibility to IDDM
(Janeway et al., 1998). |
|
Images
courtesy of Janeway C, Travers P, Walport M, Capra J.Immunobiology:the Immune System in Health and Disease.4th ed.
London Current
Biology Publication;1999. p 493.
|
back
to top of page
Environmental Causes of ß
Cell Autoimmunity and Diabetes
Other research indicates that there must be an interaction of genes and environment
to initiate the disease process (Slavkin et al., 1999). Gene-environment interactions are thought to operate in early childhood to initiate the disease process. The
exact nature of these multiple gene-environment interactions and the possible involvement of infectious and noninfectious agents is not
yet completely understood (Slavkin et al., 1999). However, we do know that several
environmental factors are associated with the onset of diabetes.
Viruses: Viral infections appear to initiate
autoimmunity in some circumstances. Islet cell antibodies and antibodies
against insulin have been detected after mumps, rubella, measles, chickenpox,
Coxsackie, and ECHO4 infections. Additionally, fetuses and newborn babies
may be at an increased risk because of their heightened likelihood of developing
persistent infections (Rewers et al., 1997). One report indicates that ß
cell autoimmunity and diabetes may be related to expression of human endogenous
retrovirus and that the mechanism may function via superantigen activation of
autoreactive T cells. However, these results remain unconfirmed (Rewers et
al., 1997)
Factors in utero: Recent evidence also points to
the probability that ß cell autoimmunity and diabetes may be a result of
enteroviral infections, which may be acquired in utero. Researchers hypothesize
that molecular mimicry may exist between the P2-C protein of Coxsackie virus and
the GAD protein and that this relationship may be responsible for ß cell
autoimmunity (Rewers et al., 1997).
Cows' milk: As discussed previously, a
controversial hypothesis that cows' milk may lead to the initiation of ß cell
autoimmunity and diabetes. A recent study, The Diabetes Autoimmunity Study
in the Young (DAISY), found that there was no association between early exposure
to cow's milk and ß cell autoimmunity in young children (Rewers et al.,
1997). However, other research has indicated that children with diabetes
are 60% more likely to have been exposed early to cow's milk than children
without diabetes (Rewers et al., 1997).
back
to top of page
Possible Prevention/Treatments for
Patients with IDDM
While the complications associated with
diabetes are severe and possibly life-threatening, improved understanding of
this IDDM has provided researchers with much optimism that this autoimmune
disease will soon be either preventable or curable (Delves et al.,
1998). Our current situation provides hope for the development
of preventative vaccines. Some researchers suggest that it may be possible
to design recombinant vaccines that would result in long-term protection against
diabetogenic strains while preventing adverse effects. Other researchers
suggest vaccinations involving antiviral agents (Rewers et al., 1997). As
evidenced by the vagueness of these possible vaccinations, much work is still
yet to be done in this area before a definitive preventative measure is
developed.
Other researchers are
targeting the relatives of IDDM patients in order to prevent the onset of type 1
diabetes. A recent pilot trial gave rise to a widespread unmasked trial in
which first and second degree relatives who test positive for islet cell
antibodies receive low doses of oral insulin. The results of this trial
will be known in 2002 (Rewers et al., 1997).
Faustman, researcher
at Harvard University, has discovered a method of correcting the MHC class I
defect and thereby preventing the T cells from reacting against autoantigens
within the ß cells (Faustman et al., 1995). Her technique includes
transplantation of one of the two Tap genes (Tap 1 or Tap 2) from normal MHC
class 1 molecules into the defective MHC class I molecules of patients with IDDM.
In vitro studies revealed that the gene-altered MHC class I molecules were,
indeed, able to elicit a normal response from CD8 T cells (Faustman et al.,
1995). Her ideas and techniques have not yet been applied to human
studies.
A less invasive
method of preventing type 1 diabetes is the administration of vitamin D. A
recent European study has demonstrated that treatment of NOD mice with the
active form of vitamin D prevented the development of insulitis (Dahlquist et
al., 1999).
Additionally, in a questionnaire and interview study that they conducted, these
researchers found that children taking vitamin D supplements were significantly
less likely to develop IDDM. They hypothesize that vitamin D played a role
in increasing tolerance to ß cells and improving sensitivity to
apoptosis. These aspects lead to a better elimination of self-reactive
effector cells (Dahlquist et al., 1999)
The usual
treatment for type 1 diabetes is insulin injections, which provide the diabetic
individual with temporary insulin that will then allow sugar to pass into their
cells. However, for those that have suffered the consequences of type 1 diabetes
for many years, full pancreas transplants may be a more permanent solution by
providing the diabetic individual
with functioning ß cells. But, as with any transplant, the body's
natural immune system may attack this foreign organ. Thus in pancreas
transplants immunosuppressive drugs must be given. These drugs are
generally accompanied by several adverse side effects including increased susceptibility to infections and even cancer (McCarren 1996). As an
alternative treatment, the Diabetes Research Institute (DRI) has recently made
significant progress in the development of the islet transplant. Recently,
Dr. Camillo Ricordi of the DRI invented an automated method of islet cell
isolation, which made it possible for scientists to obtain large numbers of
islets from a human pancreas (Diabetes 1999). Further improvements on his isolation
technique made it possible for scientists to isolate enough islets from one
pancreas to transplant into one recipient patient. Through an islet cell
transplant, type 1 diabetic patients are able to reclaim the insulin-producing ß cells
that were mistakenly destroyed by their own immune system (Diabetes 1999). This procedure is currently entering large scale clinical
trials and may
lend much encouragement to researchers looking for a treatment for IDDM.
However, with this new procedure, as with full pancreas transplants,
immunosuppressive drugs must be given and therefore the risk of subsequent
infection and cancer are still present. Click here to link to the Diabetes
Research Institute home page and view an amazing interactive animation of the
islet isolation process: http://www.drinet.org/html/ricordi_method_of_islet_isolat.htm.
Also from this page, download the appropriate plug-ins and view actual footage from an islet cell transplant in progress.
In order to provide treatment for the diabetic without life-long
immunosuppression, researchers at the University of Miami have invented a
technique by which they propose to give recipients islet cells and bone marrow
from the same donor (McCarren 1996). In this procedure islet cells and bone marrow
will be given
separately. The islet cells will originate in a cadaver donor and will
drip through a catheter into the liver of the recipient. It is hoped that
once these islet cells reach the liver they will settle in and begin to secrete
insulin. Immunosuppressive drugs will be given only initially in order to
prevent rejection of these islet cells. Five days and then eleven days
after the introduction of the islet cells, the patient will receive a bone
marrow infusion from the same donor. In this procedure the researchers are
hoping that the donated bone marrow will decrease the body's tendency to attack
the donor islet cells. Their technique is based on the theory that the
donor bone marrow will "educate" the recipient's immune system to
accept islet cells from the donor. It has been questioned whether the
donor's immune system would then attack the rest of the recipient's
organs. However, researchers at Miami suggest that although the mechanism
whereby this procedure works is unknown, the two immune systems do, indeed,
learn to co-exist (McCarren 1996). What is not yet known is whether the
islet cells will continue to survive after the stopping the use of
immunosuppressive drugs.
Thus, the
scientific community is hopeful that better preventative measures or a treatment
for diabetes can and will be successfully developed in the future.
Continuing studies in the area of IDDM will focus even more on the genetics
behind this disorder, the mechanism of autoimmunity, and the cytokines involved
in this process. The physiological effects of IDDM are severe and the
better that scientists can understand the mechanism of this disease, the better
they will be at intervening in the disease process and finding a way to prevent
or treat type 1 diabetes mellitus.
back
to top of page
References
Andersen
O. Secondary Diabetes: The Spectrum of the Diabetic Syndromes. New York: Raven
Press; 1980. p 409 - 419.
Balasa B,
Davies J, Lee J, Good A, Yeung B, Sarvetnick N. 1998. IL-10 impacts
autoimmune diabetes via a CD8+ T cell pathway circumventing the requirement for
CD4+ T and B lymphocytes. Journal of Immunology 161: 6963 - 6969.
Baldeon
M, Chun T, Gaskins R. 1998 July. Interferon alpha and interferon
gamma differentially affect pancreatic ß cell phenotype and function.
American Journal of Cellular Physiology 275(1): C25-C32.
Dahlquist, G. 1999. Vitamin D supplement in early
childhood and risk for Type 1 (insulin-dependent) diabetes mellitus.
Diabetologia 42: 51-54.
Delves P, Roitt I (eds). 1998. Encyclopedia of Immunology. 2nd Ed. San Diego: Academic Press.
Diabetes Research Institute. 1999.
Islet Cell Transplantation. <http://www.drinet.org/html/islet_cell_transplantation.htm>
and <http://www.drinet.org/html/ricordi_method_of_islet_isolat.htm.>
Accessed 2000 Apr 19.
Faustman D, Wang F. 1995 Nov 3. Harvard Medical
School: The Teacher is to Blame. <http://www.med.harvard.edu/publications/Focus/complete_texts/Nov3_1995_complete.html>
Accessed 2000 Apr 17.
InteliHealth - Home to Johns Hopkins Health Information: American Autoimmune Related
Diseases Association. 1999 Dec 27. <http://www.cfs.inform.dk/Rheumatologi/autoimmun.html>
Accessed 2000 Apr 17.
Janeway CA, Travers P, Walport M, Capra JD. Immunobiology: the Immune System in Health and Disease. 4th ed. London: Current
Biology Publication; 1999. p 493 - 494, 508.
McCarren M. 1996 June. American Diabetes
Association: Did Somebody Say Cure? <http://www.diabetes.org/diabetesforecast/96june/curehtm.htm>
Accessed 2000 Apr. 17.
Protein Data
Bank. Structure Explorer - 1ILK. <http://www.rcsb.org/pdb/cgi/explore.cgi?pid=4444956328262&page=0&pdbId=1BEN>
Accessed 2000 Apr 20.
Rewers M, Klingensmith G. 1997. Prevention of Type 1
Diabetes. Diabetes Spectrum. Vol 10:4. p 282 - 292. <http://www.diabetes.org/DiabetesSpectrum/97v10n4/pg282.htm>
Accessed 2000 Apr 18.
Scriver C, Beaudet A, Sly W, Valle D. The Metabolic and
Molecular Bases of Inherited Disease. 7th ed. New York: McGraw-Hill Inc.;
1995. p 859 - 863.
Slavkin, H. 1999 May 11. Insights on Human Health:
Towards a Common Theme in Autoimmunity. National Institute for Dental and
Cranofacial Research. <http://www.nidcr.nih.gov/slavkin/slav0499.htm>
Accessed 2000 Apr 17.
Trembleau
S, Penna G, Gregori S, Chapman H, Serreze D, Magram J, Adorini L. 1999.
Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese
diabetic mice. Journal of Immunology 163: 2960 - 2968.
Vallance-Owen
J (ed). Diabetes: Its Physiological and Biochemical Basis.
Baltimore: University Park Press; 1975. p. 1.
back
to top of page
Return back to Stacy's Immunology Home
Page
Return to the Immunology Home Page.
Return to Davidson College Biology Department Home
Page
Return To Biology Course Materials
© Copyright 2000 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: stspolnik@davidson.edu





