*this website was created as an undergraduate project for Bio307:Immunology at Davidson College*
By Amy Reid
Introduction
Fas (aka CD95 aka APO-1) is the coolest protein ever. Upon binding FasL, Fas transduces a signal that makes the cell kill itself! Fas is involved in two main events in the immune system: cytotoxic T cell mediated death of virally infected cells (here Fas would be on the virally infected cell) and apoptosis of lymphocytes in the periphery to decrease the immune response once the pathogen has been effectively terminated in the host. Fas mutations confer autoimmunity and increased lymphocyte populations in the periphery.
Fas Structure
Figure 1. This is the structure of the Fas death domain. The Fas death domain is made up of 6 alpha helicies. Helix 3 binds the FADD death domain at the start of the signal transduction. Figure courtesy of the online Protein Data Bank
The mature Fas protein is a 319 amino acid structure composed of 3 domains: an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular domain is made up of 157 amino acids and is rich in cysteine residues. The transmembrane domain is 17 amino acids long. The cytoplasmic domain is 145 amino acids long. (Huang et al, 1996)
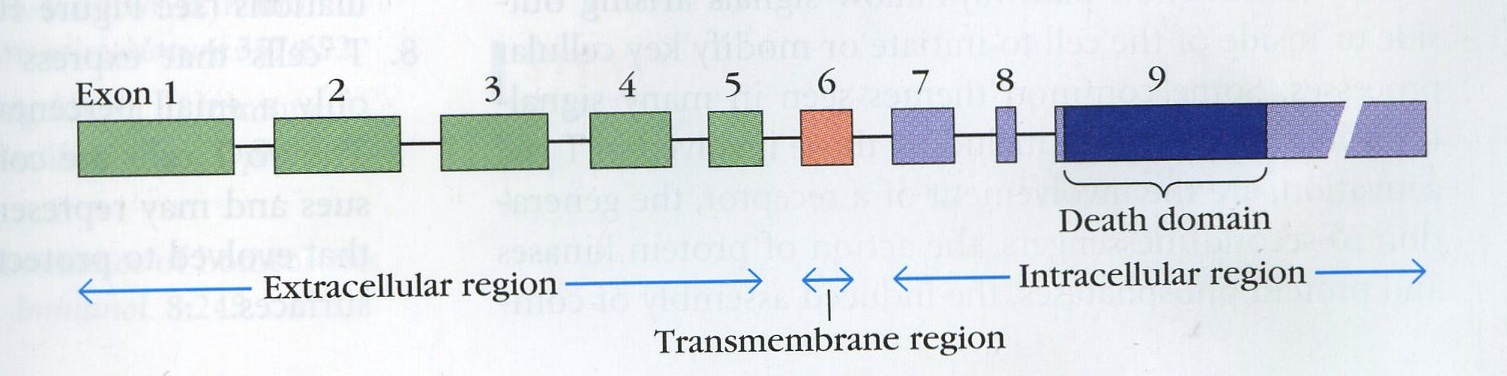
In humans, the Fas gene is on chromosome 10's long arm. The gene is found on chromosome 19 in mice. The Fas gene consists of 9 exons (Nagata & Golstein, 1995).
Figure 2. The map of the Fas locus. Exons 1 through 5 encode the extracelluar region. Exon 6 encodes the transmembrane region. Exons 7-9 encode the intracellular region. The cut shown depicts where a possible mutation could be, near the death domain, that would prevent a Fas/FasL from transducing a signal. (Goldsby et al, 2002)
Where is Fas expressed?
Fas is a member of the TNFR family (tumor necrosis factor receptor) of receptors. that is widely expressed on the surface of many different kinds of cells. High Fas expression is found on activated T lymphocytes (Janeway et al, 2000) and TNF-α and IFN -γ increase Fas expression (Nagata & Golstein, 1995). Mutations in the death domain of Fas affect the expression of a functional Fas, the consequences of this will be addressed in the section below "What happens if Fas is nonfunctional?"
Fas/FasL Signal Transduction
First, the trimerization of Fas is required to propogate a signal (Krammer, 2000).
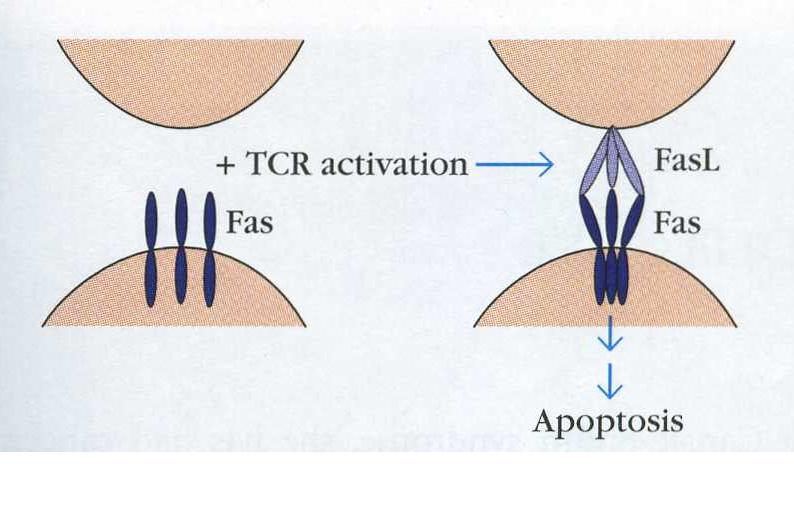
Figure 3. Fas is found on the surface of cells as a monomer. Upon T cell activation, trimerization is induced and the signal can be transduced to kill the T cell. The T cell is on the bottom, a Fas ligand bearing cell is on the top. (Goldsby, Kindt & Osborne, 2000)
Next, the signaling pathway begins.
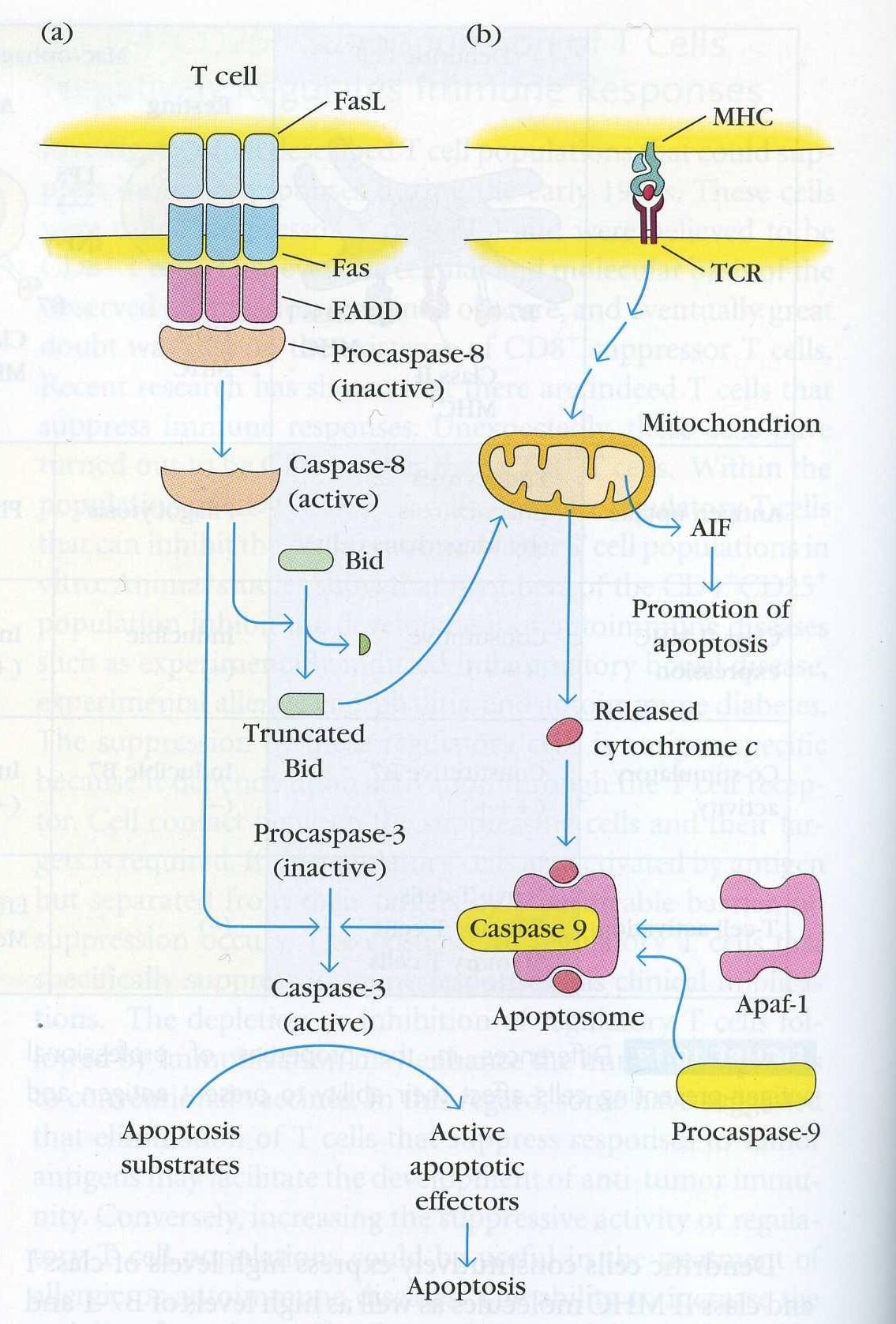
Figure 4. The signal pathways that lead to apoptosis of a T cell. A. The Fas induced pathway described in depth below. This can occur via Fas/FasL interactions on the same cell or on two different cells. This pathway also affects the second path shown in part B that does not directly use Fas to kill T cells. (Goldsby et al, 2003)
Fas/FasL binding transduces a signal by the following steps:
~FasL (on a cytotoxic T cell for example) binds Fas on the target cell.
~Fas trimerizes on the target cell surface.
~The DISC - the death inducing signalling complex -is formed in seconds after Fas/FasL binding. This refers to the complex of proteins that associates with Fas intracellularly.
~First, FADD - fas associated death domain, an adaptor protein - binds via its death domain to the Fas death domain.
~On the other end, the DED -death effector domain- of FADD binds the DED of procaspase 8 into the DISC.
~Procaspase 8 is activated and caspase 8 dissociates from the DISC and moves into the cytoplasm
~Caspase 8 cleaves procaspase 3 into active caspase 3.
(Krammer, 2000)
~Caspase 3 cleaves I-CAD. I-CAD no longer inhibits the activity of CAD.
~CAD goes into the nucleus and cuts the DNA into 180bp fragments.
(Janeway et al, 2005)
The end result of the signal pathway, whether through caspases or mitochondrial swelling and cytochrome c release, is I-CAD cutting nuclear DNA. Although, to kill a cell using the Fas/FasL system, neither a nucleus nor DNA cleavage is necessary. These two seem to be secondary to the break up of the cytoplasm which is necessary. (Nagata & Golstein, 1995).
The chemical changes resulting from the signal transduction in the cell lead to morphological changes discussed in "Fas & Apoptosis".
Where can the signal pathway be interupted?
Bcl-2 & its binding protein BAG1 inhibit apoptosis by preventing swelling of the mitochondria so no cytochrome c is released (Nagata & Golstein, 1995). Bax acts against Bcl-2 to promote apoptosis (Goldsby, Kindt & Osborne, 2000).
Various caspases promote apoptosis so molecules that target these to render them ineffective would prevent apoptosis.
Fas Function in the Immune System
Fas & Apoptosis
Apoptosis can result from one of two scenarios: the cell fails to receive survival signal so apoptosis occurs, or in the case of Fas-mediated apoptosis, there is an active signal to induce programmed cell death. (Krammer, 2000)
A cell undergoing apoptosis experiences cytoskeletal changes that lead to membrane blebbing, chromatin condensation and a break down of DNA. In the end, the cell is in many membrane bound pieces called apoptotic bodies that are phagocytosed by macrophages. In contrast to necrosis, the toxic contents of the cell do not spill out and no inflammatory response is initiated. (Goldsby et al, 2000)
The question in the late 1980s was: do Fas/Fas antibody interactions actually induce apoptosis or is the antibody to Fas just blocking growth factors from binding to transmit a signal? The question was answered by doing a southern blot of the DNA of cells in which Fas bound the antibody. The researchers saw many bands - that is the DNA had been cut. Membrane blebbing was observed as well as nuclear fragmentation and chromatin condensation. This led to the conclusion that Fas/FasL induces apoptosis. Unlike other factors that promote or inhibit apoptosis like Bcl-x, Fas initiates apoptosis. (Itoh et al, 1991)
Do Fas/FasL interactions account for the apoptosis of cells during positive and negative selection?
Positive and negative selection in the thymus is normal is lpr mice - mice with non functional Fas proteins. Therefore, Fas does not affect this system. (Nagata & Golstein, 1995).
In the case of positive selection, when T cells are being educated in the thymus to recognize self MHC, T cells that fail this selection die by lack of survival signal. Thus, Fas mediated apoptosis does not play a role here.
In the case of negative selection, mutant lpr and gld mice that have nonfunctional Fas answered this question. Researchers did not observe an affected T cell repertoire in these mice, therefore negative selection still occured as it does in wild type mice. Thus, Fas/FasL interactions do not initiate apoptosis during negative selection.
Regarding B cells, the situation is not so clear, however it is thought that apoptosis involves the mitochondrial pathway (Fig. 3b).
(Kramer, 2000)
Fas and cytotoxic CD8 T cells: killing infected target cells
When an activated cytotoxic T cell recognizes its specific antigen peptide in the context of MHC Class I, cytotoxic T cells kill the virally infected cell. T cells kill the target cell in two ways: one, by the release of toxic perforins and granzymes and two, by the engagement of T cell FasL with Fas on the target cell. Fas amplifies the granzyme/perforin pathway within target cells just as it amplifies the second pathway for killing T cells in figure 3b (Goldsby et al, 2003).
Researchers know that Fas-mediated apoptosis is involved in this process becuase mice defective for granzyme/perfornin release could still kill cells when cells expressed Fas and FasL. These mice could not kill cells that lacked Fas/FasL. Thus these two mechanisms seem to be the only two ways by which cytotoxic T cells kill their targets. (Goldsby et al, 2003).
Fas and peripheral T cell populations
Activated T cells coexpress Fas and FasL (Ju et al, 1995). This allows the T cells to be sensitive to FasL either on the same cell or on another cell. Activating B cells induces the expression of Fas, making them susceptipble to cell death via the Fas system. In mice and in humans with wild type Fas, Fas mediated cell death eliminates self reactive T cells in the periphery and activated T cells after the immune response has done its job (Nagata & Golstein, 1995).
elimination of self reactive T cells
While T cells are exposed to self antigen during education in the thymus, not every possible self protein peptide is presented; it is possible for self reactive T cells to make it out of the thymus into the periphery. These cells are actively killed by the Fas system. Researchers know Fas plays a role in deleting self reactive cells because a lack of Fas function confers autoimmunity. (Puck & Straus, 2004)
elimination of activated T cells
The immune system works by controlling the number of cells through growth and death. Part of the reason why the immune system is so successful is due to overproduction of cells and then selective killing of cells to leave the very best ones. Apoptosis via the Fas/FasL system is necessary to decrease the activated lymphocyte population in the periphery after the immune response has killed off the pathogen. (Krammer, 2000)
Fas Mutations
lpr mice
Mice that have a non-functional Fas protein are termed lpr mice because one observes lymphoproliferation in these mutants. Fas cannot transduce a signal in these mice becuase a transposable element has inserted itself into the second intron of the Fas gene. Normally, upon activation of B and T cells Fas expression is induced, however these mice will not have functional Fas on B or T cells. As a result of this loss of function mutation, researchers observed an increase in the number of active T cells, an increase in the number of IgGs and IgMs as a result of the increased B cell population, and an increase in the number of double negative T cells (Nagata & Golstein, 1995). The increased double negative T cell population could reflect the need to anergize cells that would be constantly activated (Bettinardi et al, 1997). One can infer from these data that lymphoproliferation in these mice is a result of the lack of Fas mediated apoptosis (these observations were not seen in control mice with wild type Fas). These mice also display autoimmune syndromes from which one can conclude that the Fas system is needed to kill self reactive lymphocyte populations in the periphery. (Puck & Straus, 2004)
These data point to the fact that while cytotoxic T cells have two ways of killing their targets, Fas-mediated apoptosis and the granzyme/perforin pathway, lymphocyte cell death in the periphery is wholey dependent on the Fas system.
ALPS in humans
ALPS stands for autoimmune lymphoproliferation syndrome. Patients with this disorder display many of the same symptoms as lpr mice. Investigation into the genomes of these patients revealed a variety of Fas mutations. One patient had a deletion that led to a frameshift which rendered Fas non-funtional. Another patient had a single mutation which substituted an essential base in the death domain of Fas consequently preventing the transduction of a signal. Patients with one base different from healthy individuals in the Fas gene acquire autoimmune syndromes. These data support the idea that Fas is necessary to delete self reactive T lymphocytes. Furthermore, patients had increased B cell populations which conferred impaired humoral immunity. (Fisher et al, 1995)
Studying the family tree of affected individuals, researchers found that impaired Fas function was not fully responsible for ALPS: parents who had the same mutation as their affected children did not have symptoms of ALPS (Fisher et al, 1995). Further studies have revealed that ALPS can also result from FasL mutations as well as caspase 8 mutations, however 75% of those with ALPS do have Fas mutations (Puck & Straus, 2004).
Fas and HIV/AIDS
HIV is a virus that causes the disease AIDS in which the host's immune system is compromised. Cytotoxic T cells whose T cell receptor is specific for HIV peptides are three times more susceptible to apoptosis induced by Fas compared to cytotoxic T cells specific for other antigens. In addition, macrophages infected with HIV can initiate the apoptosis of HIV-specific cytotoxic T cells in part through the Fas mediated system. These concepts together lead one to hypothesize that the effeciency, proliferation and survival of HIV-specific cytotoxic T cells is likely imparied. (Mueller et al, 2001)
The population of CD4+ cells in an AIDS patient is very low compared to a healthy indvidual meaning that these particular cells are killed more often than they should be. The products that HIV produces make Fas-mediated apoptosis easier. It is likely that Fas plays a role in the depletion of the CD4+ T cell population. (Krammer, 2000)
Fas and T. cruzi
Trypanosoma cruzi is a parasite that infects inhabitants of Latin America leading to Chagas' disease. The parasite infects the host and to continue living inside the cells, T. cruzi prevents apoptosis. Researchers observed an inhibition of Fas mediated apoptosis specifically. In preventing apoptosis of the host cells, the parasite is able to continue living off of the nutrients the cell provides. To block apoptosis, the parasite in its amastigote stage (the cell division stage once the cell has been infected) blocks caspase 8 and so the apoptotic signal delivered by Fas/FasL binding is arrested. T. cruzi lives in the host cells by blocking the signaling pathway of Fas-mediated apoptosis. (Nakajima-Shimada J et al, 2000)
Impaired Fas function plays a role in various diseases and syndromes as demonstrated above. This speaks to the fact that Fas-mediated apoptosis in the immune system plays a large role in the homeostasis of the body and in the basic function of the immune system - the regulation of cell population size. The various syndromes are treated with drugs that treat the symptoms. I am unaware of any drugs that bind Fas.
Sources
Bettinardi A, Brugnoni D, Quiros-Roldan E, Malagoli A, La Grutta S, Correra A, Notarangelo LD. Missense Mutations in the Fas Gene Resulting in Autoimmune Lymphoproliferative Syndrome: A Molecular and Immunological Analysis. 1997 Feb 1;89(3):902-09.
Centers for Disease Control and Prevention: National Center for Infectious Diseases, Division of Parasitic Diseases. 23 Sep 2004. Chagas Disease Fact Sheet. <http://www.cdc.gov/ncidod/dpd/parasites/chagasdisease/factsht_chagas_disease.htm#where> Accessed 16 Mar 2006.
Fisher GH, Rosenburg FJ, Straus SE, Dale JK, Middelton LA, Lin AY, Struber W, Lenardo MJ, Puck JM. Dominant Interfering Fas Gene Muations Impair Apoptosis in a Human Autoimmune Lymphoproliferative Syndrome. Cell 1995 Jun 16;81:935-46.
Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Immunology. 5th ed. New York: W.H. Freeman and Company; 2003. chapters 1, 14, and 10.
Goldsby RA, Kindt TJ, Osborne BA. Immunology. 4th ed. New York: W.H. Freeman and Company; 2000. chapters 10 and 2.
Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR Structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 1996 Dec 19-26;384(6610):638-41.
Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushuma S-I, Sameshima M, Hase A, Seto Y, Nagata S. The Polypeptide Encoded by the cDNA for Human Cell Surface Antigen Fas can mediate apoptosis. Cell 1991 July 26; 66:233-43.
Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6th ed. CITY: Garland Science Publishing; 2005. chapters 8 and 6.
Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL Interactions Required for Programmed Cell Death after T Cell Activation. Nature 1995 Feb 2;373:444-48.
Krammer PH. CD95's Deadly Mission in the Immune System. Nature 2000 Oct 12;407:789-95.
Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-Induced Apoptosis of HIV-Specific CD8+ Cells. Immunity 2001 Dec;15:871-82.
Nagata S, Golstein P. The Fas Death Factor. Science 1995 Mar 10;267:1449-56.
Nakajuma-Shimada J, Zou C, Masatoshi T, Umeda M, Nara T, Aoki T. Inhibition of Fas-mediated apoptosis by Trypanosoma cruzi infection. Biochimica et Biophysica Acta 2000;1475:175-83.
National Human Genome Research Institute, NIH. <http://research.nhgri.nih.gov/alps/> Accessed 16 Mar 2006.
Puck JM, Straus SE. Somatic Mutations - Not Just for Cancer Anymore. New England Journal of Medicine 2004 Sep 30;351(14):1388-1390.