Mononucleosis, also commonly called mono and the kising disease, is an infection most frequently spread through contact with saliva of an infected individual. The disease is caused by an infection by the Epstein-Barr virus, which infects the B-cells of the immune system. The Epstein-Barr virus, also known as EBV and human herpesvirus 4, is a member of the herpesvirus family. As many as 95% of adults in the United States between 35-40 have been infected with EBV at some point in their lives. EBV infections during adolescence have been known to cause infectious mononucleosis 35-50% of the time. It is also known that EBV establishes a lifelong latent infection in the B-cells of the immune system. This latent infection has been linked to Burkitt's lymphoma and nasopharengeal carcinoma. EBV appears to play a role in, but is not the sole cause, of these cancers (Schmid 2005).
The Epstein-Barr virus is spread through saliva and infects the B-cells of the immune system. This infection is initiated by the binding of viral the the virus' glycoprotein, gp350/220, to complement receptor 2, CR2 or CD21 (Prota et al. 2002). CR2 is a membrane protein on B-cells that is involved in transmitting growth promoting signals to the interior of the cell. It is a part of the B-cell co-receptor and associated with cross-linking and can increase signal up to 1000-10,000 times the normal strength. Thus, the binding of the viral protein to CD21 leads to B-cell acitvation and proliferation, thus leading to the production of more viral protein. This in turn leads to the activation and proliferation of CD8 T-cells cspecific for the viral antigen and excess mononuclear white blood cells, thus giving the disease its name. The infection will eventually, usually after a few weeks, be cleared by cytotoxic CD8 T-cells. However, once the lytic infection is cleared the latent virus still remains in B-cells(Janeway et al.).
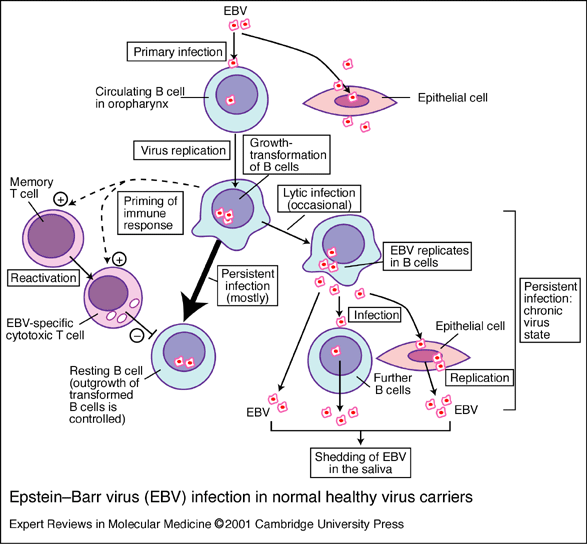
Figure.1 This image provides the basics for the primary pathway of infection by EBV (Murray et al. 2001). *permission pending*
The Lytic Infection
In the lytic infection the virus follows the path discussed above and continues to replicate. The virus activates specific proteins that act to help the virus replicate and contribute to the proliferation of the infected B-cells. One major protein associated with the lytic infection of EBV is the ZEBRA protein. This protein has a function that is unique in herpesvirus function. The ZEBRA protein is responsible for the disruption of the latent cycle and the induction of the lytic cycle in EBV. ZEBRA is a transcriptional activator which binds to elements within EBV promoters (Baumann et al. 1995). This results in increased replication of the viral genome as well as the viral antigens (Chan et al. 1988). Another essential protein that is used by EBV is BCRF-1. BCRF-1 is a viral homolog of IL-10. IL-10 serves to upregulate T-helper-2 cells and downregulates T-helper-1 cells. T-helper-1 cells are the primary cells involve in the inflamatory response. Thus, by the use of BCRF-1 to downregulate T-helper-1 cells helps EBV avoid clearing by these cells in spite of increased viral antigen production (Hemond). Other proteins that have been shown to be involved in the lymphoproliferation associated with the lytic infection are the G protein-coupled receptors (GPCRs) encoded by BILF-1 genes of EBV. A study by Biesser et al. in 2005 shows that the GCPRs encoded by these genes ae not only functional but activate transcription factors CRE and NFκB. Both of these factors are involved in the transcription of proteins involved in the survival signal, thus leading to further lymphoproliferation. However, in a non-immunocompromised individual the lytic infection will eventually be cleared though depending on the strength of the infection this clearing may take weeks (Biesser et al. 2005).
The Latent Infection
Latently infected B-cells do not express normal viral antigen, but instead express viral protein EBNA-1. EBNA-1 plays an essential role in maintiaining the viral genome, but its importance in latently infected B-cells comes from its interaction with proteasomes that prevents the degredation, thus preventing presentation by MHC class 1 molecules and the destruction of the host cells (Janeway et al). Some studies have also linked EBNA-1 to malignant transformation, leading to malignincies such as Burkitt's lymphoma and nasopharengeal carsinoma. A study by Humme et al. in 2003 showed a marked decrease in transformation of B-cell lines to immortal cell lines, which is equivalent to tumorigenesis in vitro, in cells infected by a strain of EBV virus lacking the genes coding for the EBNA-1 protein (Janeway et al.). Malignant tumor spreading has also been tied to the latent membrane proteins LMP2A and LMP2B. Though the mechanisms by which the LMP2 proteins promote cell motility is not clear, but a study has suguested thaat the two proteins may directly engage signaling pathways that affect cell adhesion and motility (Allen etal. 2004).
Diagnosis of Mononucleosis
The vast majority of cases of infectious mononucleosis can be identified by the presence of pharengitis (sore throat), lymphadenopathy (swollen lymphnodes, usually on the neck), and fever persisting for up to 4 weeks. Serologic test in a diagnosis of infectious mononucleosis will show an elevated white blood cell count, an increased total number of lymphocytes, greater than 10% atypical lymphocytes, and a positive reaction to a mono spot test, which detects primarily IgM (immunoglobulin of isotype M) heterophile antibodies. High levels of heterophile antibodies are typically observed during the first month of infection and these levels rapidly decline after week 4 (CDC 2005). False-positive results may be found in a small number of patients and are usually attributed to and infection by Rubella, Hepatitis, Systemic lupus or erythematosus (SLE) (Werner 2005). False-negative tests occur in about 10-15% of patients and are most common in children younger than ten years of age (CDC 2005).
Treatment of Mononucleosis
Like most viral infections, treatment for infectious mononucleosis is primarily to alleviate symptoms. The most commonly reccomended treatment for the disease is REST! The use of anti-inflamatories and fever reducers, such as Ibuproufen, are commonly used to allieviate the swelling and aches assosciated with mononucleosis. However, these have no effect on the duration of the symptoms. Acyclovir is a drug that competitively bloack DNA polymerase, thus inhibiting viral DNA synthesis. Treatment of EBV infections with acyclovir has been shown to reduce the oralpharengeal shedding of the virus, but the duration of the symptoms was no different from the group of patients treated with a placebo (Nieves 1998).
Allen M, Young L, Dawson C. The Epstein-Barr Virus-Encoded LMP2A and LMP2B Proteins Promote Epethelial Cell Spreading and Motility. Journal of Virology. Feb 2005 Vol. 79, No. 3, 1789-1802.
Baumann R, Warren G, Askovic S. Restoration of the Epstein-Barr virus ZEBRA protein's capacity to disrupt latency by the addition of heterologous activation regions. Virology. Aug 1, 1995 Vol. 211, No. 1, 64-72.
Beisser P, Verzijl D, Gruijthuijsen Y, Beuken E, Smit M, Leurs R, Bruggeman C, Vink C. The Epstein-Barr Virus BILF1 Gene Encodes a G Protein-Coupled Receptor That Inhibits Phosphorylation of RNA-Dependent Protein Kinase. Journal of Virology. Jan 2005 Vol. 79, No. 1, 441-449.
Chan, M Y Soo, Y Y Gan, A Fones-Tan, P S Sim, A Kaur, C T Chew. Epstein Barr Virus (EBV) Serology in the Dianosis of NPC- Comparison Between IFA and Two Commercial ELISA Kits. Singapore Medical Journal 1990 <http://www.sma.org.sg/smj/3906/articles/3906ra1.html> Accessed 2006 Apr 24.
Hemond K. Modulation of the Host Cytokine Network. 1999. <http://www.brown.edu/Courses/Bio_160/Projects1999/ies/cytok.html> Accessed 2006 Apr 24.
Humme, Gilbert Reisbach, Regina Feederle, Henri-Jacques Delecluse, Kristine Bousset, Wolfgang Hammerschmidt, Aloys Schepers. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proceedings of the National Academy of Sciences of the United States of America. Sept 16, 2003 Vol 100, No. 19, 10989-10994.
Janeway, A.C, P. Travers, M. Walport., J.M. Shlomchik.2005. Immunobiology: The Immune System in Health and Disease. 6th ed. New York, NY: Garland Publishing. pp 228-229.
Nieves M. “What every pediatrician should no about Infectious Mononucleosis.” 1998. The Pediatric Bulletin. <http://home.coqui.net/myrna/mono.htm> Accessed 2006 Apr 24.
Prota, D. R. Sage, T. Stehle, and J.D. Fingeroth. The crystal structure of human CD21: Implications for Epstein–Barr virus and C3d binding. Proceedings of the National Academy of Sciences of the United States of America. Aug 6, 2002 Vol. 99, No. 16, 10641-10646.
Schmid D. "Epstein-Barr Virus and Infectious Mononucleosis."
2005. National Center for Infectious Diseases. <http://www.cdc.gov/ncidod/diseases/ebv.htm>
Accessed 2006 Apr 20.
Werner K. "Mononucleosis spot test." 2005. MedlinePlus Medical Encyclopedia. <http://www.nlm.nih.gov/medlineplus/ency/article/003454.htm> Accessed 2006 Apr 24.