
This web page was produced as an assignment for an undergraduate course at Davidson College.
African Trypanosomiasis
Commonly referred to as sleeping sickness, African trypanosomiasis is a disease that results from an infection with the extracellular, flagellar parasite, Trypanosoma brucei. Two variants of T. brucei exist in distinct regions of Africa, T. b. gambiense of West Africa and T. b. rhodesiense of East Africa, but they cause basically similar forms of the disease. T. brucei can survive in both humans and tsetse flies, and thus can be transmitted between these two hosts. If an tsetse fly infected with T. brucei bites a human, cycles of chronic infection typically results in the human because these parasites possess genetically programmed mechanisms for continuous antigenic variation. African trypanosomiasis is related to, but distinct from American trypanosomiasis (Chagas' disease).
Symptoms
Symptoms of East and West African trypanosomiasis are similar. Both forms of the disease begin with relatively minor signs, but they become increasingly severe if the infection persists and progresses throughout the body. The first sign of the disease is a painful sore from a tsetse fly bite that becomes red and otherwise visibly infected after 1-2 weeks. Indications of disease can then spread to the rest of the body over the next weeks, months, and possibly years. Such symptoms include: fever, general swelling, lymph node swelling (especially at the back of the neck), rash, weight loss, headaches, muscle and joint aches, and fatigue. If left untreated, severe affects on the CNS will be seen as the parasite infects CNS tissues. Indeed, personality changes, confusion, slurred speech, inability to concentrate, difficulty walking, and seizures may occur in late stages of infection. Patients with advanced African trypanosomiasis often sleep during the day, while suffering insomnia at night (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006).
Epidemiology
The tsetse fly lives in vegetation and waters of the savanna and woodland regions in equatorial Africa (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006). Thus, there exists a "sleeping sickness" belt (Fig 1). Indeed, rates of African trypanosomiasis are highest in the regions of West, East, and Central Africa where tsetse flies and humans co-exist. As mentioned before, the specific type of T. brucei that infects the tsetse fly/human hosts varies by region: T. b. rhodesiense is found primarily in East Africa (Botswana, Ethiopia, Kenya, Malawi, Tanzania, Uganda, Zaire, and Zimbabwe), while T. b. gambiense is found in areas of Central and West Africa. Each year the World Health Organization receives reports of between 25,000 and 40,000 combined cases of East and West African trypanosomiasis. Unfortunately, however, because African Trypanosomiasis mainly affects poor populations in rural areas, perhaps most cases are not unreported (Gubler DJ, 1998). Therefore, the actual number of individuals suffering from the African trypanosomiasis may be 100,000 per year or more (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006). Until wars end and wide-spread, effective public health campaigns are put into effect, it is likely that frequent and devastating epidemics of African trypanosomiasis will sweep through Central Africa (Gubler DJ, 1998). It is important to note that tourists in Africa rarely come into contact with the tsetse fly, unless they spend long period of time in rural areas.

Figure 1. The modern distribution of tsetse flies in Africa. Attested flies and thus incidence of African Trypanosomiasis is highest in the orange regions of this map of Africa. Image courtesy of: (Sept J. 1999), permission pending.
Transmission and Structure of a T. brucei
The T. brucei are protozoa that exists in distinct stages depending on host (Fig 2). T. brucei multiply in the gut of tsetse flies and travel to the salivary glands, where they take on the epimostigote and later, the metacyclic form. As the metacyclic form, the protozoa can pass from the saliva of the fly into the bloodstream of a human. In the blood and also extracellular tissues, T. brucei generally exist and divide as the long, slender form (Fig 3). Note the evidence of flagellae in the long, slender form of the T. brucei (Fig 4), which have been determined to be essential for viability of the parasites in the human bloodstream (Broadhead R, et al., 2006). A small number of T. brucei also exist in the non-dividing short, stumpy form that can be passed back to fly hosts as the cycle of transmissions between fly/human continues (Fig 2).
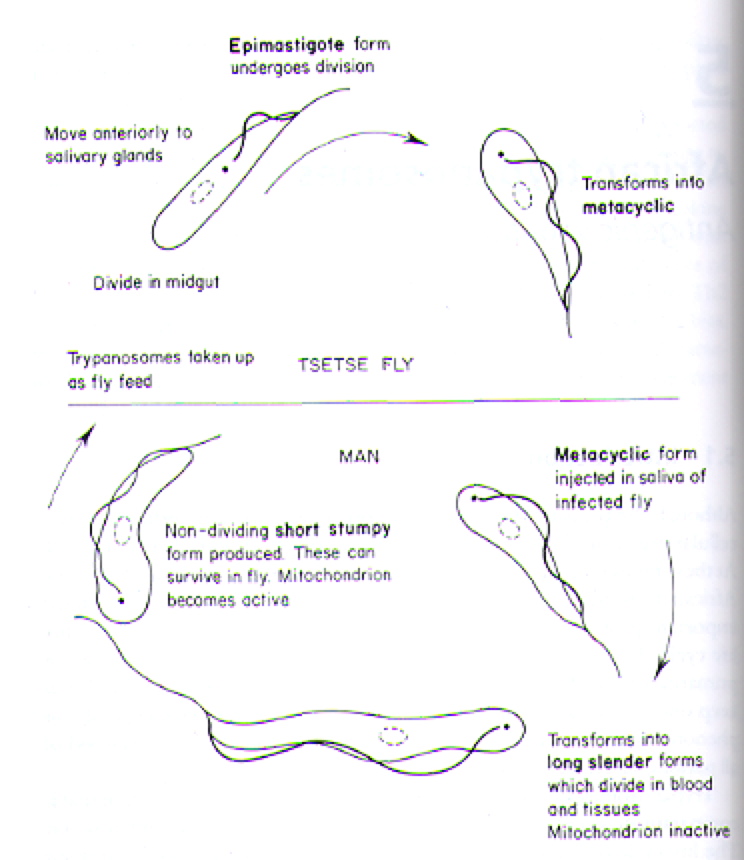
Figure 2. Cartoon illustrating the lifecycle of T. brucei in its alternative hosts, the tsetse fly and man (human). Image courtesy of: (Wakelin D, 1996).
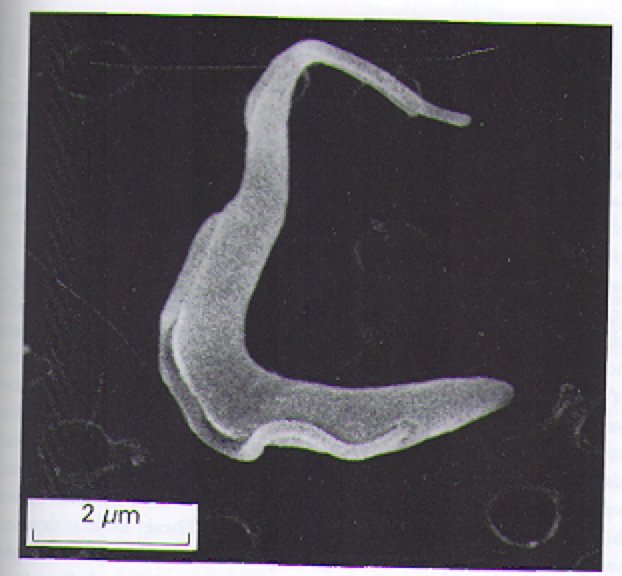
Figure 3. Scanning electron micrograph image of the long, slender form of T. brucei. This the predominant form of T. brucei found in the bloodstream and extracellular tissues of infected humans. Image courtesy of: (Wakelin D, 1996), citing Dr. L. Tetley and Professor K. Vickerman.
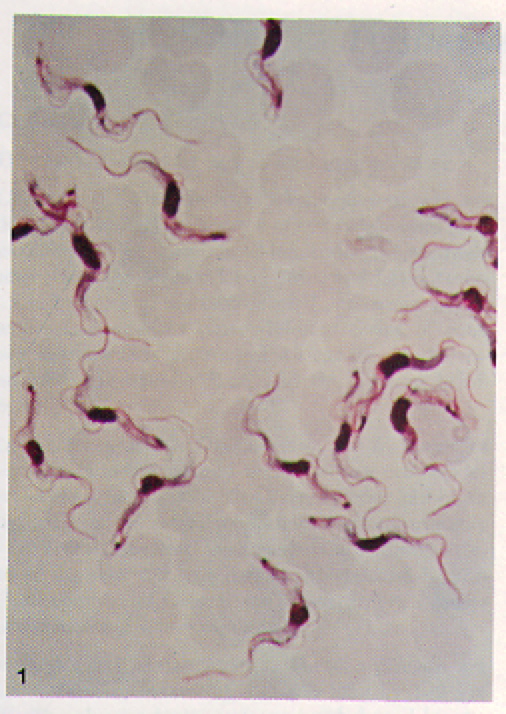
Figure 4. Blood film containing many long, slender T. b. gambiense. Note the visible motile, flagella on individual T. b. gambiense organisms. T. b. rhodesiense are visibly indistinguishable from T. b. gambiense at this level of magnification. Image courtesy of: (Ash LR, et al., 1984).
The out ser surface of T. brucei parasites is covered with a tightly packed layer of glycoproteins called Variant Surface Glycoproteins (VSGs) (Fig 5 b and c). The structure motif of VSGs has been described in detail (Blum ML, et al., 2003), but generally they are heteropolymers of polysaccharides attached to proteins anchored in the plasma membrane (Fig 6) (Taylor ME, et al., 2003). Feel free to explore the generic structural motif of a VSG using the JMOL image in Figure 7. Usually, only one specific VSG is expressed on the surface of a parasite at a time. The VSGs prevent the cells of the host from directly accessing the plasma membrane of the parasite and provide a means for antigenic variation (Wakelin D, 1996).
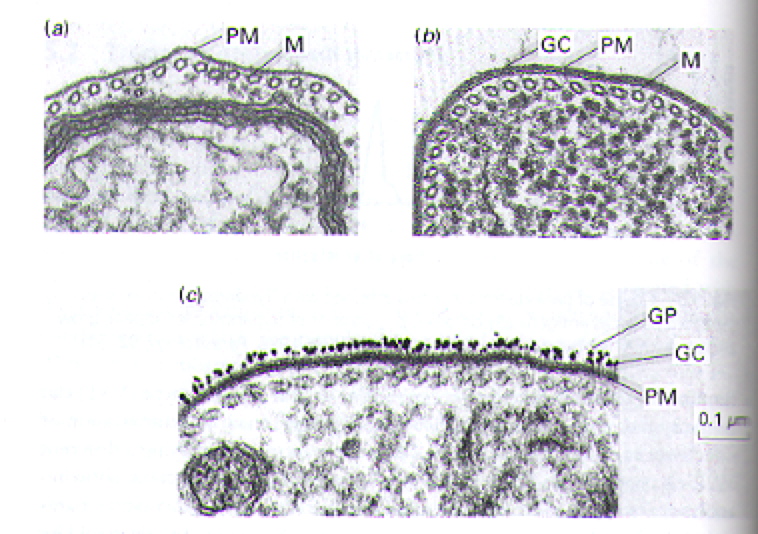
Figure 5. Transmission electron micrographs highlighting the layer (or lack of layer) of glycoproteins that cover the surface of T. brucei. For comparison, panel a represents a close-up portion of a single parasite in which the glycoprotein coat has been removed. Panel b shows the intact, visible glycoprotein coat on another close-up image of a single parasite. Panel c illustrates the highlights the surface of a parasite in which the glycoprotein coat was labeled with gold particles (i.e. the black specs). Several abbreviations are used to draw attention to key portions of the images: PM= plasma membrane, M= microtubules, GC= glycoprotein coat, GP= gold particles. Image courtesy of (Wakelin D, 1996), citing Dr. L. Tetley and Professor K. Vickerman.
Figure 6. Cartoon of VSGs anchored into a small portion the plasma membrane of T. brucei outer surface. Logically, the VSG coated plasma membrane comprises the entire outer surface of a parasite. Image courtesy of: (Taylor ME, et al., 2003).
Figure 7. JMOL image of structural motif of VSG. Image courtesy of Protein Data Bank and (Blum ML, et al., 2003).
Pathology
T. brucei effectively evade the human immune system by a fascinating genetically programmed system of antigenic variation. When even a single parasite crosses the epithelial layer via the bite of an infected tsetse fly, local pain and inflammation result as the innate immune system responds to the invader. Nevertheless,when innate, non-specific mechanisms of destruction are not sufficient to clear the pathogen (as is often the case in humans), the parasite grows and multiples in the bloodstream, while an primary adaptive immune response is built. Indeed, the adaptive response is evident by the appearance of redness and swelling at the site of infection 1-2 weeks after the tsetse fly bite. VSGs on the surface of the parasite are recognized as foreign antigens by specific BCRs and TCRs. Generally a highly specific B-cell mediated antibody response is generated against to certain VSG epitopes. The adaptive immune response eventually kills all of the clones of the original parasite via antibody-mediated adaptive immune response. However, some parasites spontaneously change the VSG coating by altering which VSG gen is expression. This process is known as gene conversion. In fact, there exist 806 different genes that encode for VSGs (Berriman M, et al., 2005). Each VSG gene encodes for structurally similar, but extremely unique VSGs. Because different VSGs are distinct enough that they are each seen as representative of 'new' pathogens by the immune system, a separate, primary adaptive immune response must be generated to clear the parasites with new VSGs. The time required to mount a sufficient primary adaptive immune response allows the parasite to divide, multiply, and occasionally change its VSG surface again. Thus, although theoretically all T. brucei can eventually be cleared by the human immune system, spontaneous changes in which VSG is expressed allow the parasite to stay one step ahead of the adaptive immune system. Indeed, patients with African trypanosomiasis suffer from chronic cycles of infection (Fig 8). The disease progresses as these cycles lead to chronic inflammation, fever, and the build-up of immune complexes. Furthermore, the severe and often irreversible neurological damage occurs as parasites reaches deeper and into less protected areas of the body, such as the brain. Fatigue is probably a result of the body constantly funneling of resources to fight a never ending infection.
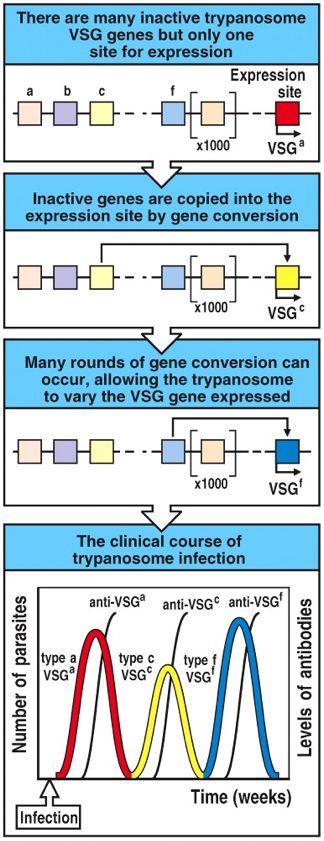
Figure 8. Cartoon illustrating the relationship between VSG gene conversion of T. brucei (first three panels) and the resulting chronic cycles of infection (last panel). Image courtesy of: (Janeway CA, et al., 2005).
Diagnosis
African trypanosomiasis is typically diagnosed first according to symptoms and then through a positive blood test for presence of T. brucei. Cerebrospinal fluid samples, lymph, and skin tissue samples can also be tested for T. brucei (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006). ELISA methods, fluorescent antibody, and/or serum IgM levels may be used to assess the presence of T. brucei. Samples should be gathered from individuals during the febrile periods to assure that there will be adequate levels of T. brucei for detection (Ash LR, et al., 1984).
Treatment
Often, hospitalization is necessary to effectively treat African Trypanosomiasis because the administration of IV antiprotozoal agents is required (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006). Individuals in the early stages of T. b. gambiense infection (West African trypanosomiasis) are prescribed Pentamidine isethionate or Suramin. Likewise, those suffering from early stages of T. b. rhodesiense (East African trypanosomiasis), should receive Suramin (Drugs for Parasitic Infections, 2004). Suramin is a inhibitor of protein-tyrosine phosphatases and thus interferes with the ability of the parasite to function (Zhang YL, et al., 1998). Indeed, both Pentamidine isethionate and Suramin serve to disrupt metabolic pathways within the parasites, causing them to fail to replicate, die, and eventually be cleared by the immune system.
When individuals present with more advanced stages of African Trypanosomiasis, especially with signs of CNS damage, they are prescribed Melarsoprol or Eflornithine (Drugs for Parasitic Infections, 2004). These medications also work by disrupting cellular processes within the parasites, but are are stronger antiprotozoal agents. Melaroprol is extremely toxic (to both the parasite and the host) and causes numerous side effects in the host. Eflornithine is often prohibitively expensive.
Because of the cost, toxicity, and potential for T. brucei to develop resistance to currently available antiprotozoal agents, scientists are investigating the other treatments for African Trypanosomiasis. For example, investigators have attempted to find RNA aptamers active against T. brucei with some limited success (Goringer HU, et al., 2006).
If left untreated, African Trypanosomiasis can be fatal. Individuals should promptly seek their healthcare provider if they have additional questions or if they suspect they have contracted African Trypanosomiasis.
Prevention
At the present, no vaccines or medications exist to prevent African trypanosomiasis. Nevertheless, much like insect bites can be avoided to prevent malaria, contact with tsetse flies and African trypanosomiasis can be prevented by several means, including pesticide application and common sense. The CDC recommends several precautions to avoid attracting and prevent bites from tsetse flies when in Africa: 1) wear thick in weight, light and bland in colored clothing that covers your entire body, 2) wear insect repellant and reapply it often, 3) sleep with a netting around your bed, 4) check closed areas/spaces (such as vehicles) for insects before entering, 5) avoid rides in open-air moving vehicles and dust created by them, and 6) stay away bushes and shrubbery, where tsetse flies may hide (West African Trypanosomiasis Fact Sheet, 2004; East African Trypanosomiasis Fact Sheet, 2006).
For more information, you can visit:
The Center for Disease Control: Division of Parasitic Diseases
References
Ash LR, Orihel TC. 1984. Atlas of human parasitology. 2nd Edition. Chicago: Ame Soc of Clin Path P.
Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-Sayed NM. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309: 416-22.
Blum ML, Down JA, Gurnett AM, Carrington M, Turner MJ, Wiley DC. 1993. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature 362: 603-9.
Broadhead R, Dawe HR, Farr H, Friffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440: 224-7.
Drugs for Parasitic Infections. 2004 Aug. The Med Lett: 1-12.
East African Trypanosomiasis Fact Sheet. 2006. The Center for Disease Control, Division of Parasitic Diseases. <http://www.cdc.gov/ncidod/dpd/parasites/trypanosomiasis/factsht_ea_trypanosomiasis.htm>. Accessed 2006 Apr 25.
Goringer HU, Homann M, Zacharias M, Adler A. 2006. RNA aptamers as potential pharmaceuticals against infections with african trypanosomes. Hand Experi Pharm 173: 375-393.
Grubler DJ. 1998. Resurgent vector-borne diseases as a global health problem. Emerging Infectious Diseases 4. <http://www.cdc.gov/ncidod/eid/vol4no3/gubler.htm>. Accessed 2006 Apr 25.
Janeway CA, Travers P, Walport M, Shlomchik MJ. 2005. Immunobiology: The immune system in health and disease, 6th edition. New York: Garland Publishing.
Sept J. 1999. Tsetse fly modern distribution. University of Indiana: Anthropology P380 Africa info website. <http://www.indiana.edu/~origins/images/tsetsemap.jpg>. Accessed 2006 Apr 25.
Taylor ME, Drickamer K. 2003. Introduction to glycobiology. Oxford: Oxford UP.
Wakelin D. 1996. Immunity to parasites: how parasitic infections are controlled. 2nd Edition. Cambridge: Cambridge UP.
West African Trypanosomiasis Fact Sheet. 2004. The Center for Disease Control, Division of Parasitic Diseases. <http://www.cdc.gov/ncidod/dpd/parasites/trypanosomiasis/factsht_wa_trypanosomiasis.htm>. Accessed 2006 Apr 25.
Zhang YL, Keng YF, Zhao Y, Wu L, Zhang ZY. 1998. Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases. J Biol Chem 273: 12281-12287.
Return to Jackie's Immunology Main Page