Insulin
________________________________________________________________________________________________
This web page was produced as an assignment for an undergraduate course at Davidson College
________________________________________________________________________________________________
Discovered in 1921 by Sir Frederick Grant Banting and Charles Herbert Best, insulin is a protein in the human body that plays a key role in the metabolism of carbohydrates as it is involved in one part of the process, which converts food into fuel (Kane and Rivers). This web page will provide a description of insulin focusing specifically on its structure, synthesis and action, function, mutations and orthologs.
The Structure of Insulin
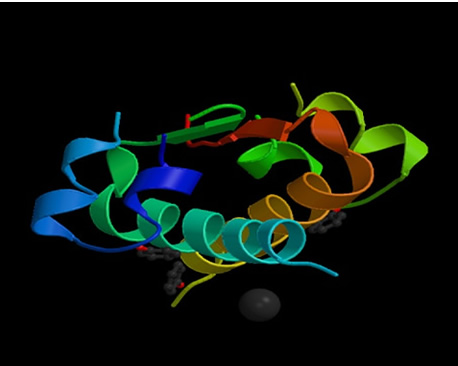
Insulin, in is native structure, is constructed of two peptide
chains, referred to as A and B, which are held together by two disulfide bonds
between the cysteine amino acids. Due to the collection of carbon-rich amino
acids (i.e.: leucine and isoleucine) in the center of the protein, insulin has
a hydrophobic center, which is then surrounded on the outside by hydrophilic,
charged amino acids (i.e.: arginine, glutamine and lysine) (Goodsell, 2001).
This arrangement provides insulin with a very stable protein structure. With
a molecular weight of only 6000 Daltons, the protein itself is not very large
(Bowen, 1999). Click to see a chime
image of the insulin, so that you can view a 3-D model of the molecule and
move it around yourself.

Figure 1: Structure of human insulin hexamer in complex with phenol (Whittingham, 1998). http://www.rcsb.org/pdb/cgi/explore.cgi?job=graphics&pdbId=1ZEG&page=0&pid=111981047618785
Synthesis of Insulin
Insulin is manufactured in considerable amounts by the beta cells of the pancreatic islets of Langerhans, a small section of the pancreas (Kane and Rivers). It is very difficult for small proteins to fold into stable structures, so to avoid this problem larger precursors of insulin are synthesized first (Goodsell, 2001). Preproinsulin, the first precursor, is generated as a single chain when the insulin mRNA is translated. Once introduced into the cisternae of the endoplasmic reticulum, the signal peptide is deleted, creating proinsulin (King). Proinsulin differs from insulin in that it has a third peptide, C, which connects the A and B peptides together. This C peptide, however, is spliced from the chain by several endopeptidases found in the endoplasmic reticulum, creating the active form of insulin.
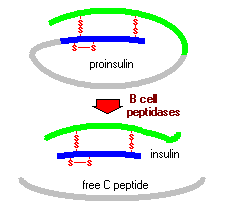
Figure 2: This figure shows the actions of peptidases in modifiying proinsulin to insulin (Bowen, 1999). http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin.html
In the Golgi, the insulin (along with the lone C peptide) is enclosed within secretory granules, folded into its native structure and then the disulfide bonds are created to secure it in this conformation (King). The secretory granules then gather in the cytoplasm. The insulin remains here until the beta cells are stimulated, at which point exocytosis occurs and the insulin disperses into the pancreatic capillaries. Although it has no known biological function, the C peptide is also released into the blood stream as well (Bowen, 1999).
Insulin Secretion
Secretion of insulin primarily occurs in response to increased concentrations
of glucose in the blood, but the exact mechanism remains unclear. However,
there are several characteristics of the process that have been repeatedly
shown. First, glucose enters the beta cells through glucose transporters (see
Carbohydrate metabolism below) by means of facilitated diffusion. As a result
increased glucose concentrations outside the cell lead to increased glucose
concentrations
inside
the cell.
Second, membrane depolarization of the beta cells occurs due to the heighten
levels of glucose. This in turn causes extracellular calcium to rush into the
cell, which is thought to be a stimulus for the export of the secretory granules
(containing insulin) out of the cell. Third, calcium-independent pathways are
triggered with elevated glucose levels in the beta cells (Bowen, 1999).
The Actions of Insulin
Once insulin is secreted by the pancreas, it goes directly to the liver via the portal vein where it has profound effects on carbohydrate and lipid metabolism. The insulin receptors, which are integral membrane proteins, are activated (King). The insulin receptor has two alpha subunits, which are completely extracellular and contain the binding site for insulin. Attached to each of the alpha subunits by sulfur bonds are two beta subunits, which extend through the membrane and aid in anchoring the protein to the cell wall. The two alpha-beta complexes are connected to each other by disulfide bonds (Kane and Rivers).
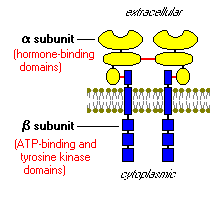
Figure 3: This is adrawing of the insulin receptor showing the two alpha-beta complexes. The alpha subunits areconnected to their respective beta unit through a sulfur bond, while the two complexes are attached to each other via a disulfide bond (both bonds shown in red) (Bowen, 2000). http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html
This is considered a tyrosine kinase receptor because it serves as an enzyme, which moves phosphate groups from ATP to tyrosine residues on intracellular target proteins (Bowen, 2000). When insulin binds to the alpha subunit, it results in autophosphorylation of the beta subunits (they phosphorylate themselves) on the tyrosine residue. Now the catalytic activity of the receptor is stimulated causing a cascade effect, which phosphorylates numerous enzymes, such as insulin receptor substrate 1(IRS-1), creating a complex biological response. IRS-1, the most well known of the phosphorylated substrates of the insulin receptor, is involved in signaling and stimulating other enzymes that operate further down the chain (Bowen, 2000).
Carbohydrate Metabolism
During digestion in the small intestine, glucose is released from carbohydrates (ie: sucrose or starch) through the process of hydrolysis and then diffuses into the blood stream. With the increase of glucose in the blood, insulin is secreted from the beta cells and helps cells throughout the body accept, utilize and store glucose. Glucose metabolism differs though depending on the type of cell being targeted by insulin (Bowen, 2000).
Muscle, Adipose, and Other Tissues
These tissue types require the use of glucose transporters (a common type is
GLUT4), which is only made available in the presence of insulin. Under normal
conditions cannot be accessed, and therefore cannot transport glucose because
the glucose transporters are found in cytoplasmic vesicles underneath the
service of the plasma membrane. However, once insulin binds to the receptors,
these vesicles fuse with the plasma membrane and the glucose transporters
penetrate through the plasma membrane. At this point, glucose can be transported
into the cell at a rapid rate. Once blood levels return to normal conditions,
insulin leaves the receptors, and the glucose transporters are moved back
into the cytoplasm (Bowen, 2000). Watch an animation of this process.
Liver Tissue
When glucose is released from carbohydrates in the small intestines a substantial
portion goes directly to the cells of the liver, so that it can be changed
from glucose to glycogen. Glycogen, a storage polymer, is synthesized when
the enzyme hexokinase is triggered, which phosphorylates glucose, preventing
it from exiting the cell. In addition, other enzymes, such as phosphofructokinase
and glycogen synthase, that aid in the conversion of glucose to glycogen
are activated by insulin (Bowen, 2000). In terms of hepatic glucose homeostasis,
there is an increase in the amount of sugar absorbed by the liver (glucose
storage in form of glycogen) and a decrease in the amount of sugar released
from the liver. Therefore, the overall effect is a decrease in blood sugar
(King).
Lipid Metabolism
In general, insulin not only encourages the use of carbohydrates over fatty acids for energy, but it also indirectly results in the accumulation of fat in the adipose tissue. Because the metabolism of lipids and carbohydrates are interrelated, insulin also has an effect on lipid metabolism.
Liver Tissue
If glycogen starts to build up to high levels in the liver (approximately 5%
of the liver mass), the liver will repress additional synthesis. So when
more glucose continues to enter the liver cells, it is redirected into a
different metabolic pathway. Within this pathway, fatty acids are made and
then transported out of the liver as lipoproteins. While circulating these
lipoproteins are broken apart into free-floating fatty acids that are utilized
in other tissues. Of these tissues, adipocytes, in particular, use the fatty
acids in order to make triglycerides (Bowen, 2000).
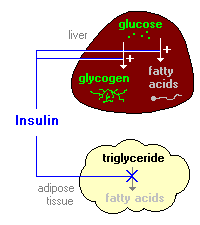
Figure 4: Diagram depicting insulin's influence on the liver producing fatty acids and inhibiting the break down of fat in adipose tissue (Bowen, 2000). http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html
Adipose Tissue
Insulin prohibits lipase from functioning, so the triglycerides cannot be hydrolyzed
into fatty acids. In this way insulin prevents the breakdown of fat in adipose
tissue. In addition, when insulin is present, glucose can enter into the
adipocytes, where it is then used to make glycerol. This glycerol along with
fatty acids, which are transported from the liver, triglycerides are synthesized,
allowing for further build up of fat in the tissue (Bowen, 2000). In short,
it triggers lipogenesis and inhibits lipolysis (King).
Additional Functions of Insulin
In addition to its role in carbohydrate and lipid metabolism, insulin serves the following functions (King):
· Increases amino acid transport into cells- when insulin levels are low, proteins are degraded
· Regulates transcription, changing the amount mRNA present in the cell
· Activates cell growth, DNA synthesis and cell replication
· Increases the permeability of most cells to potassium, magnesium and phosphate ions
Mutants of Insulin and Their Effects on the Body
Insulin plays an important role in regulating carbohydrate and lipid metabolism, and so if there is a mutation in the structure of insulin itself or along any step of the pathway that insulin is involved, it can have detrimental effects. Since there is no one common mutation, here is a list to learn more about the different types of mutations concerning insulin that can occur. Most often these mutations lead to an insufficiency or an overabundance of insulin, which are known as diabetes mellitus or hyperinsulinemia respectively (Bowen, 2000). Diabetes mellitus has been categorized into two major types, insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM), according to the cause of the disease (King).
Type 1 or Insulin-dependent diabetes mellitus
This type of diabetes affects about 2 million people in the United States.
It is usually caused by an inability for the body to produce insulin or by
an autoimmune response, where the body’s own white blood cells attack
the beta cells of the pancreas, thereby destroying them. This form disease
of the disease can usually be controlled by synthetic, bovine or porcine
insulin injections (Kane and Rivers).
Type 2 or Non-Insulin-dependent diabetes mellitus
Type 2 diabetes is more common than type 1 diabetes, affecting about 85% of
the total diabetic population (Kane and Rivers). The onset of the disease
is usually during adulthood and is due to insulin resistance by the body.
For reasons that are not yet known, target tissue stop reacting to insulin.
Researchers have found a range of defects in patients from abnormal insulin
receptors to defective signaling (Bowen, 2000). However, since insulin is
still being produced and secreted normally for the most part, insulin injections
are not useful form of therapy. Most patients have problems with obesity
though because of accumulation fat in adipose tissue. Thus the major treatments
are a low carbohydrate diet and an exercise program (Kane and Rivers).
Hyperinsulinemia
Hyperinsulinemia is much less common than diabetes, and is frequently caused
by an insulin-secreting tumor or an insulin overdose. The high levels of
insulin in the body results in a drop in blood glucose levels, which can
prevent the brain from receiving enough energy. This can lead to a life-threatening
situation where the body is in insulin shock (Bowen, 2000).
Orthologs
The amino acid sequence for insulin is highly conserved among vertebrates to
point where we have been able to use bovine and porcine insulin for people
who cannot produce their own insulin (Bowen, 2000). In fact, insulin from
pigs differs by only one amino acid: threonine in humans and alanine in pigs.
Cows are also very similar, only differing by three amino acids. You can
view the highly conserved sequences for insulin among several species: human,
gorilla,
chimpanzee,
or a ground
squirrel.
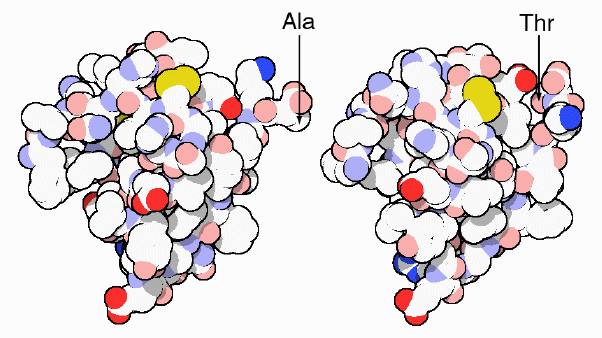
Figure 5: This is an image of the protein structure of human insulin (right) and pig insulin (left), showing their one amino acid difference (Goodsell, 2000). http://www.rcsb.org/pdb/molecules/pdb14_1.html
References
Bowen, R. March 13th, 2000. http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html
March 5th, 2003.
Bowen, R. June 15th, 1999.http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin.html
March 5th, 2003.
Goodsell, David. 2000. http://www.rcsb.org/pdb/molecules/pdb14_1.html
March 9th, 2003.
Kane, Rob and Rivers, Keith. http://chemistry.gsu.edu/CAISER/modules/inslin/insulin.html March 9th, 2003.
King, Michael. June 15th, 2001. http://www.indstate.edu/thcme/mwking/diabetes.html
March 5th, 2003.
http://www.ncbi.nlm.nih.gov/ National Center for Biotechnological Information. March 10th, 2003.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM Online Mendelian Inheritance in Man. March 9th, 2003
http://www.rcsb.org/pdb/index.html Protein Data Bank. March 10th, 2003.
________________________________________________________________________________________________
Return to Davidson's Biology Homepage