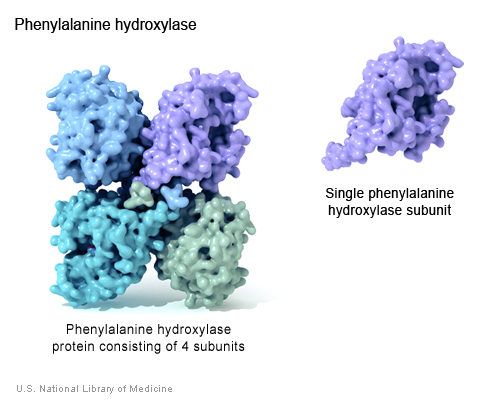
Phenylalanine Hydroxylse (PheOH) is an enzyme coded for by the PAH gene, found on human chromosome 12. PheOH is responsible for the conversion of the amino acid phenylalanine (phe) to tyrosine. It is regulated by the substrates phenylalanine, tetrahydrobiopterin, and phosphorylation (Kobe et al. 1999). PheOH has two forms, consisting of two or four identical subunits, present in equilibrium; the tetrameric form of PheOH is shown below (Kobe et al. 1999).

(Genetics Home Reference 2005 <http://ghr.nlm.nih.gov/ghr/picture/ph>)
Mutations in PAH can result in the genetic disorder Phenylketonuria (PKU), causing an increase in the plasma concentration of phe. Normal uptake of phe in one's diet is regulated by PheOH, but those with PKU have an intolerance to dietary phe because of their inability to catalyze the amino acid. Symptoms most common to PKU include high blood serum levels of phe, lighter skin and hair pigmentation, and mental retardation (OMIM 2004; <http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=261600>). While there is no cure for PKU, it can be successfully treated with a life-long restrictive phe diet and with dietary supplements. Newborn screening and prenatal testing for PKU is available (Gene Reviews 2004; <http://www.geneclinics.org/profiles/pku/details.html>).
According to Kobe, et al (1999), PheOh is regulated by phenylalanine (phe), tetrahydropiopterin (BH4), and phosphorylation. The group used a truncated dimer form of the enzyme that still "retains the catalytic and regulatory properties of the wild-type protein and crystallizes more readily" (Kobe et al. 1999).
The C-terminus of the PheOH monomer is characterized as the catalytic domain, and is also responsible for tetramerization. The PheOH active site is located in a deep pocket of helical walls within the C-terminus. The N-terminus is the regulatory domain and extends across the active site of the catalytic domain and is structurally similar to the regulatory domain of phosphoglycerate dehydrogenase, an enzyme involved in serine biosynthesis, and PCD/DCoH, involved in the regeneration of BH4 and activation of NHF1 which in turn activates PAH transcription. (Kobe et al. 1999). It is also thought that there is a region near the 111-117 amino acid sequence that hinges when the enzyme is activated and is responsible for the cleavage of phe. The figure below is a monomer of PheOH; the red indicates the C-terminus catalytic domain, the green indicates the core of the regulatory domain, and the cyan the N-terminal autoregulatory domain. The 111-117 region is green, found closest to the beginning of the red region.
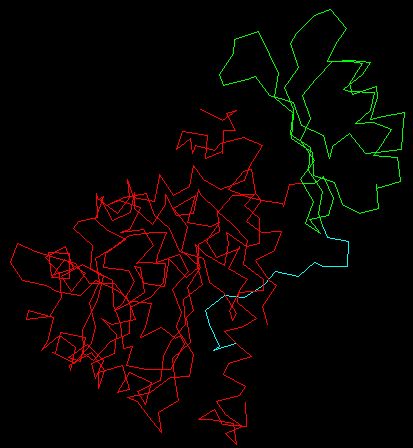
Modified QuickPDB file (PDB <http://www.rcsb.org/pdb/cgi/explore.cgi?job=graphics&pdbId=2PHM>)
Previous experimentation shows that phe is the major regulatory event. It causes a greater than one hundred-fold activation in PheOH and many structural changes (Hufton et al 1995 & Kappock & Caradonna 1996, as cited in Kobe et al 1999). Kobe et al. focuses on the structure of the phosphorylated and dephosphorylated PheOH at the N-terminus. Both phosphorylation and dephosphorylation of PheOH result in inactive states of the enzyme and there are no noticeable differences in the structures of the two forms.
Kobe et al. states that Phe is though to have two binding sites, one being a catalytic site, and one being a regulatory site. The phe binding site, near the hinge domain, may be the factor that causes the drastic shape change in the enzyme resulting in the cleavage of the phe substrate. It is important to note that PheOH will catalyze phe in the dephosphorylated state. However, there must be a high concentration of phe in order for this to occur. The phosphorylation of PheOH, which does not noticeably change the structure of PheOH, results in increased phe metabolism. Kobe et al. (1999) postulates that the N-terminus phosphorylation site may be altered when phosphorylated. Since the N-terminus covers part of the active site, there may structural changes during phosphorylation, moving the N-terminus from the active site, allowing for phe to bind to PheOH at a lower concentration. The group gives another possibility, but a less likely one: they suggest that the phosphate, when bound to the movable N-terminus, may facilitate the binding of phe to the active site.
Another aspect of PheOH regulation due to structure is found in inhibition by BH4. BH4, according to Kobe et al., may bind to PheOH near the hinge site (111-117 sequence). Kobe et al. offers two hypothesis as to why BH4 inhibits PheOH. First, BH4 may hinder the structural change at the hinge site of PheOH when Phe OH is bound with phe, resulting in the inhibition of phe catabolism. Second, BH4 may block the movement of the N-terminus phosphorylation site, resulting in a less active phe binding site (Kobe et al. 1999).
Another point that Kobe et al. touches on is mutations in PAH and their resulting structural changes in the enzyme. Many different mutations have been identified in PAH that result in reduced PheOH function and PKU. Many mutations in the PheOH active site have obvious structural implications. However, mutations in the catalytic domain and the N-terminus region have also been identified. Kobe et al.'s earlier postulates of N-terminus function and hinge site regulation function may help explain the decreased activity of mutant PAH in areas other than the active site.
Gene Reviews. 2004 Jul 8. Phenylalanine Hydroxylase Deficiency. <http://www.geneclinics.org/profiles/pku/details.html>. Accessed 2005 Feb 15.
Genetics Home Reference. 2005 Feb 11. Phenylalanine Hydroxylase. <http://ghr.nlm.nih.gov/ghr/picture/ph>. Accessed 2005 Feb 15.
Kobe B, et al. 1999 May. Structural basis of autoregulation of phenylalanine hydroxylase. Nature Structural Biology 6; 5: 442-8. Abstract available at <http://www.nature.com/cgi-taf/DynaPage.taf?file=/nsmb/journal/v6/n5/abs/nsb0599_442.html&dynoptions=doi1108519696>. Accessed 2005 Feb 9.
Online Mendelian Inheritance in Man [OMIM]. 2004, Nov 15. Phenylketonuria <http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=261600>. Accessed 2005 Feb 15.
3D representations of PheOH and PheOH mutants: <http://www.pahdb.mcgill.ca/?Topic=Information&Section=Molecular&Page=0>
PKU news: <http://www.pkunews.org/>
PKU support organization: <http://www.pkunetwork.org/>
PKU screening: <http://my.webmd.com/hw/raising_a_family/hw41965.asp>
Contact Megan at mecastle@davidson.edu