ORTHOLOGS OF HEMOGLOBIN
What are Orthologs?
The origin of similar genetic sequences that are found in different species can sometimes be traced back to speciation events resulting in divergent copies of a single gene. These similar genetic sequences are said to be orthologs, and they code for proteins which are likewise similar, but not exactly the same (Sadava et al., 2008). Orthologs are useful for identifying the relatedness of various species as well as for understanding the origin and function of the proteins they code.
Development of Hemoglobin
Found in erythrocytes, hemoglobin is well known as
the protein primarily responsible for carrying oxygen throughout the human
body. However, hemoglobin is found in a variety of other species, including plants
and bacteria, strongly suggesting that these genes were found in very early
common ancestors who then passed on divergent copies of the gene during speciation
events. In all vertebrates, the amino acid sequences coding for the α-subunits of
the hemoglobin protein and those coding for the β-subunits are 50% similar (Hardison,
1996), suggesting a common ancestry between the two. These more derived tetramers
in fact descended from the more primitive myoglobin, a monomeric protein responsible
for oxygen storage and delivery in the earliest jawed vertebrate, lampreys. Around 450 million years ago, this myoglobin
gene gave rise to the earliest hemoglobin-coding genetic sequence when it
replicated. When cartilaginous fish and bony fish diverged about 50 million
years later, the early hemoglobin gene evolved in such a way that resulted in two new forms coding for the
alpha and beta subunits, and these forms were later passed onto all vertebrates
along that evolutionary line, including mammals.
The usefulness of hemoglobin is not just recognized
by animals. Hemoglobin is also used in plants to carry oxygen for the purposes
of respiration. Leghemoglobin, a relative of hemoglobin, has also been detected
in the nodules of plants, binding to oxygen so that sensitive nitrogen-fixing
enzymes may function properly. The leghemoglobin protein sequence differs than
that of vertebrate hemoglobin by 80%, appropriately reflecting the distance in
ancestry between the two genes (Hardison, 1996). Similarly, hemoglobin and
other homologous genes have been found in algae and protozoan, which are also responsible
for trapping oxygen for the purposes of respiration. In the yeast Saccharomyces, a flavohemoprotein
consisting of a heme domain and a FAD domain is responsible for signaling and
regulation under favorable oxygen conditions (Hardison, 1996). The
flavohemoportein is also present in Escheria
coli.
These examples all highlight the status of hemoglobin as a gene with
multiple functions and as a descendant of an ancestral gene that must
have been very ancient, as similar forms of it are found in many other
different species of organisms.
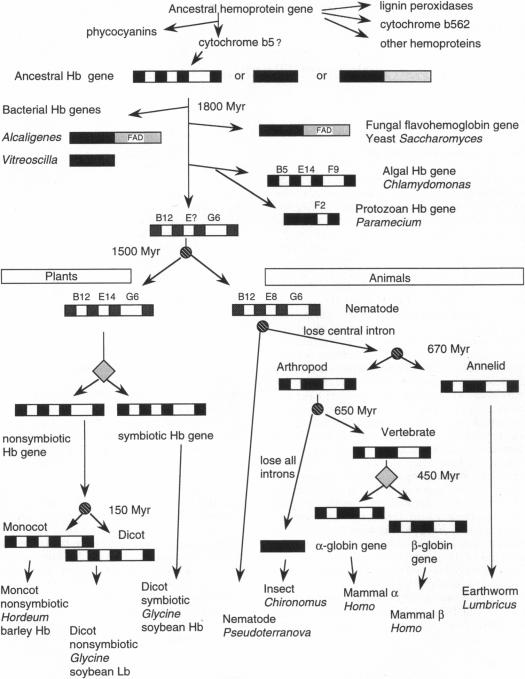
Figure 1.
Significant events in hemoglobin genes development.
Shaded boxes are extrons, open boxes are introns, and
lightly-shaded boxes show
the FAD domain. Diamonds signify gene replication events, and circles
signify
speciation events.
The location
where an intron interrupts the codon of the
amino acid chain is shown above the intron,. For example, B5 is the
amino
acid at
the fifth position of helix B. Image retreived from Hardison, 1996.
Permission pending. Link to the article here.
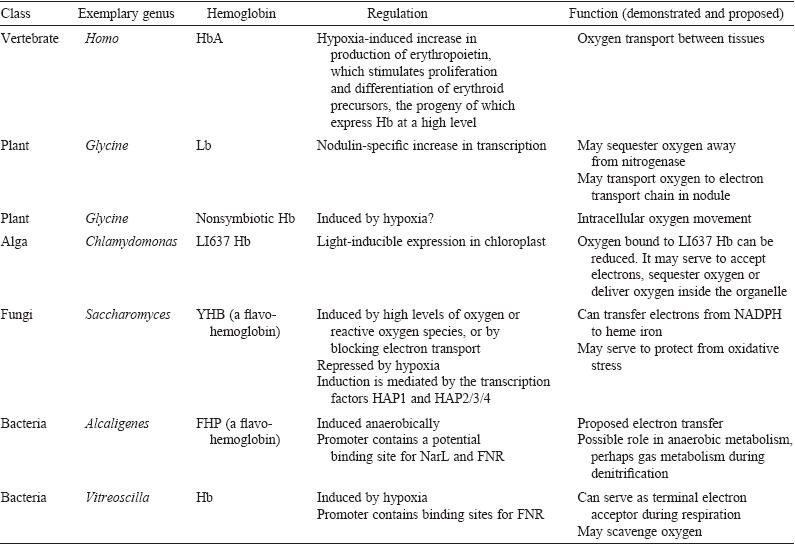
Figure 2.
Various functions of hemoglobin proteins and hemoglobin-like proteins
in different spieces of organisms. Image retreived from
Hardison, 1998. Permission
pending.
Form Follows Function
In many species, the function of hemoglobin typically involves binding to an oxygen molecule. This function is due to the highly conserved structure of the hemoglobin protein, allowing it to perform the same function in different organisms. To be so conserved, hemoglobin's function must be very important to all living organisms, since the protein is found in almost all species. This consistence in function suggests that the structures of hemoglobin proteins in different species is conserved, and thus the genetic sequences encoding these proteins must likewise be similar. Examining the subunits of hemoglobin in comparison to the ancestral myoglobin protein mentioned above will likely give some clue about which sections of the hemoglobin is most conserved and thus most important to protein shape and function.
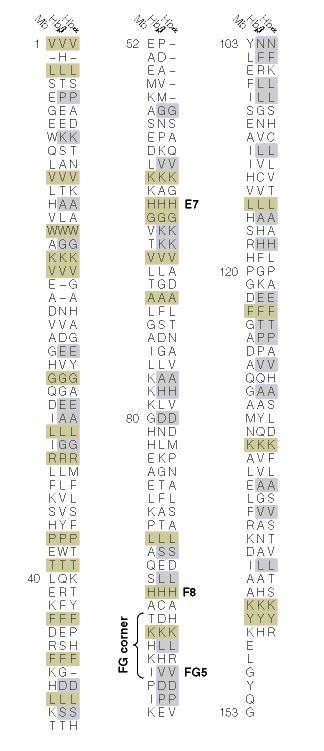
Figure 3. A comparison between the α-subunit of hemoglobin, β-subunit of
hemoglobin, and myglobin. Sequences highlighted in grey are conserved between the
α-subunit and β-subunit of hemoglobin.Sequences highlighted in light brown are c
onserved in both subunits and the ancestral myoglobin. Imaged retrieved from
http://www.aw-bc.com/mathews/ch07/c07emhp.htm. Permission pending.

Figure 4. Percentage similarity in α-subunits of hemoglobins found in various species.
Species which diverged further back in time had less similarity in their amino acid
sequences coding for the α-subunit. Image retrieved from
\http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A2. Permission pending.
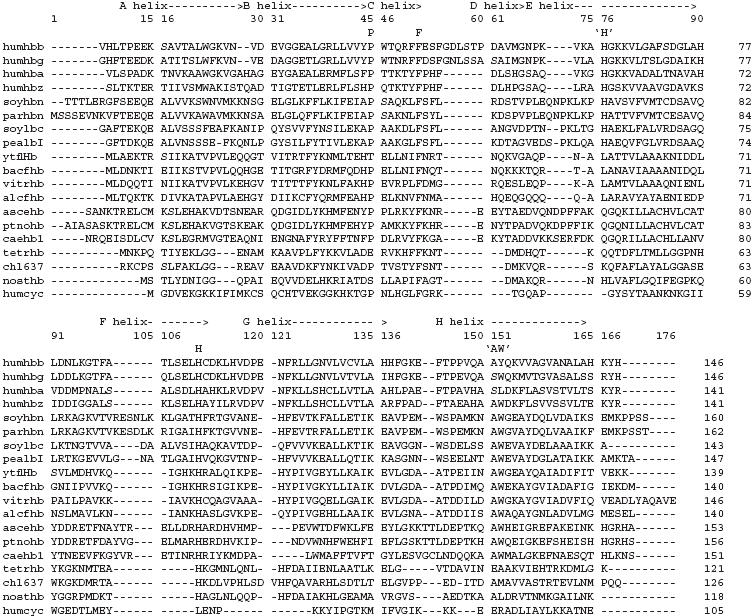
The table shown above highlights several similarities found in the hemoglobin orthologs. It is interesting to note that species that were more divergent from each other had less similarity in their amino acid sequences. Although certain amino acid sequences did vary from one another, important amino acids that are important to the function of hemoglobin genes are shown to be highly conserved, and these amino acids are seen in the columns with the letter representing the amino acid above that column. Overall, we can see that while divergence does indeed changed parts of the amino acid sequences, specific amino acids that are critical to hemoglobin function remain conserved.
WORKS CITED
Bruce
Alberts, Dennis Bray, Julian Lewis, Martin Raff, Keith Roberts, and James D.
Watson. Molecular Biology of the Cell
Fourth Edition. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A2.
March 2010.
Hardison, Ross C. A brief history of hemoglobins: Plant,
animal, protist, and bacteria. Proc.
Natl. Acad. Sci., 1996; 93: 5675-5679.
Hardison, Ross. Hemoglobins from Bacteria to Man: Evolution of Different Patterns of Gene Expression. The Journal of Experimental Biology, 1998; 1099-1117. Link to article here.
Mathews, Van Holde and Ahern. Evolution of Myoglobin/Hemoglobin Proteins. http://www.aw-bc.com/mathews/ch07/c07emhp.htm . March 2010.
Sadava D, Heller CH, Orians GH, Purves WK and Hillis DM. Life: The Science of Biology 8th Edition. Massachusetts and Virginia: Sinauer Associates Inc. and W.H. Freeman and Company, 2008. Print.
Please send suggestions, questions, or comments to tahua@davidson.edu