This web page was produced as an assignment for an undergraduate course at Davidson College.
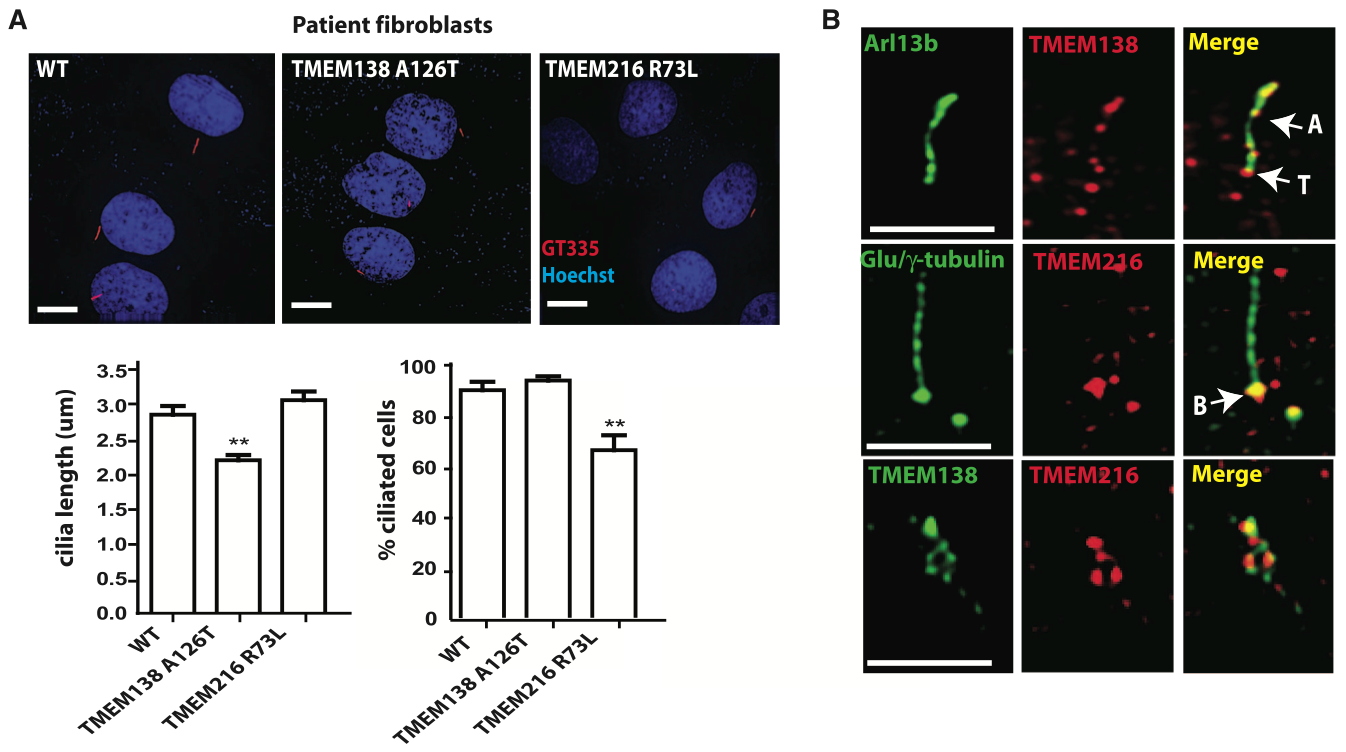
Figure 3: Cellular funcion and localization of TMEM138 and TMEM216
The co-expression of TMEM138 and TMEM216 and the congruency of the phenotypes resulting from their mutation suggested to the authors that the proteins may subserve a common function. To test this hypothesis, the authors labeled and analyzed the location of the TMEM138 and TMEM216 in human wild-type (WT) cells, cells with mutated TMEM138, and cells with mutated TMEM216.

Image from Lee et al., 2012
Panel A: The authors labeled human fibroblasts containing WT genes, mutated TMEM138, or mutated TMEM216 for cilia (red, mAb GT335 for polygutamylated tubulin; shown in images). They then measured the length of the cilia on these fibroblasts (shown in graphs). A mutation in TMEM138 resulted in significantly shorter cilia compared to fibroblasts containing WT genes or mutated TMEM216. A mutation in TMEM216 resulted in a significantly lower percentage of fibroblasts becoming ciliated compared to WT or TMEM138-mutated fibroblasts.
Panel B: The authors labeled TMEM138 and TMEM216 along with ciliary markers (Arl13b and Glu/gamma-tubulin) in WT human embryonic kidney (IMCD3) cells. The proteins were localized to adjacent but non-overlapping vesicle pools near the ciliary base (B). TMEM138 was also located in the ciliary axoneme (A) and the transition zone (T) between the base and axoneme.
Data not shown: The authors claim that additional data shown in supplementary figures demonstrate that knockdown (using small interfering (si) RNA) of TMEM216 disrupted TMEM138 vesicular movement, but TMEM138 knockdown did not impair TMEM216 vesicular movement. They also claim that data shown in other supplementary figures demonstrate that this adjacent protein localization does not occur in zebrafish.
Conclusions: The authors conclude that the proteins serve closely related ciliogenesis functions in mammals and that the function of TMEM138 is dependent on TMEM216, but the function of TMEM138 is not dependent on TMEM216. Furthermore, they conclude that this adjacent protein localization is a product of the adjacency and co-regulation of TMEM138 and TMEM216. These data prompted the authors to test next the mechanism by which TMEM138 and TMEM216 vesicles are localized and whether mutations in these proteins result in indistinguishable phenotypes in lower vertebrates lacking TMEM138 and TMEM216 adjacency and co-regulation. The results of those experiments are shown in Figure 4.
Reference:
Lee, J.H., Silhavy, J.L., Lee, J.E., Al-Gazali, L., Thomas, S., Davis, E.E., Bielas, S.L., Hill, K.J., Iannicelli, M., Brancati, F., Gabriel, S.B., Russ, C., Logan, C.V., Sharif, S.M., Bennett, C.P., Abe, M., Hildebrandt, F., Diplas, B.H., Attie-Bitach, T., Katsanis, N., Rajab, A., Koul, R., Sztriha, L., Waters, E.R., Ferro-Novick, S., Woods, C.G., Johnson, C.A., Valente, E.M., Zaki, M.S., Gleeson, J.G. 2012. Evolutionarily Assembled cis-Regulatory Module at a Human Ciliopathy Locus. Science. 335: 966-969.
Genomics Page
Biology Home Page
Email questions or comments to chford@davidson.edu
© Copyright 2011 Department of Biology, Davidson College, Davidson, NC 28035